Alpha, Beta & Gamma Radiation
This lesson covers:
- The reasons behind the instability and radioactivity of atomic nuclei
- The four primary types of nuclear radiation emissions
- The fundamental properties of alpha, beta, and gamma radiation
- The occurrence of alpha and beta decay in unstable nuclei
Nuclear instability causes radioactivity
The stability of an atomic nucleus is determined by the balance between two opposing forces:
- Strong nuclear force - This force acts to hold the protons and neutrons within the nucleus together.
- Electrostatic force - This is the force that causes positively charged protons to repel each other.
A nucleus may become unstable and thus radioactive if it has:
- Too many neutrons
- Too few neutrons
- A total number of nucleons (protons and neutrons) that is too high
- Insufficient binding energy to hold it together
This instability leads to radioactive decay, during which the nucleus emits energy in the form of radiation until it reaches a stable configuration.
The four main types of nuclear radiation
The radiation emitted by unstable nuclei can be categorised into four main types, each with distinct characteristics:
| Type | Symbol | Composition | Charge | Mass |
|---|---|---|---|---|
| Alpha | 2 protons + 2 neutrons | +2 | 4 u | |
| Beta minus | Electron | -1 | Negligible | |
| Beta plus | Positron | +1 | Negligible | |
| Gamma | Photons | 0 | Negligible |
These types of emissions are capable of ionising atoms, which means they can displace electrons from atoms or molecules, transforming them into ions.
Key radiation properties
Nuclear radiation types differ in their ionising power, speed of emission, and how far they can travel through different materials:
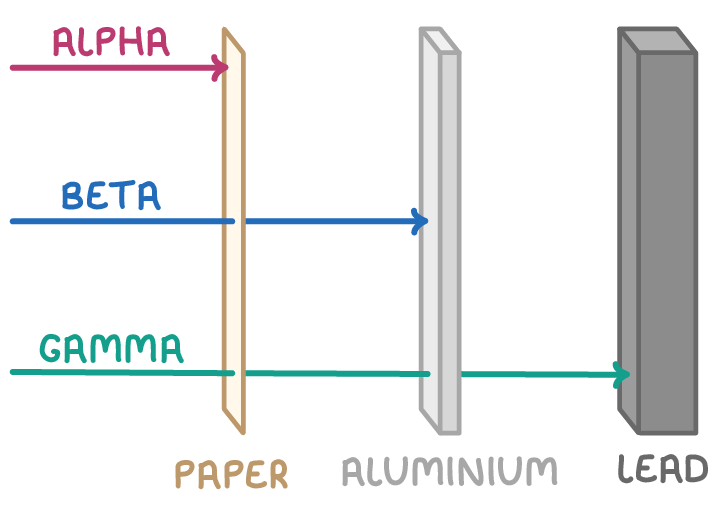
| Type | Ionising Power | Speed | Range |
|---|---|---|---|
| Alpha | High | Relatively slow | A few centimetres in air |
| Beta minus | Medium | Fast | Several millimetres in metal |
| Beta plus | Medium | Fast | A few centimetres in metal |
| Gamma | Very high | Very fast | Many centimetres in lead |
Gamma rays, in particular, possess short wavelengths and high energies, enabling them to penetrate more deeply than alpha or beta particles.
When does alpha decay occur?
Alpha decay is characteristic of very heavy nuclei, such as uranium, which have more nucleons than can be stably maintained:
For instance:
92238U→90234Th+24He
In this process, the emission of an alpha particle decreases both the atomic number and mass number of the original nucleus, leading to a more stable configuration.
When does beta-minus emission occur?
Beta minus decay typically happens in isotopes with an excess of neutrons. This process converts a neutron into a proton while emitting an electron:
75188Re→76188Os+−10e+νˉe
This transformation increases the atomic number by one, but the mass number remains unchanged, resulting in a different element.
When does beta-plus emission occur?
Beta plus decay typically happens in isotopes with an excess of protons. This process converts a proton into a neutron while emitting a positron (beta-plus particle):
This transformation decreases the atomic number by one, but the mass number remains unchanged., resulting in a different element.