Rutherford's Alpha Particle Scattering
This lesson covers:
- How ideas about atomic structure have changed over time
- Rutherford's gold foil scattering experiment
- How Rutherford used the results to propose the existence of the atomic nucleus
History of atomic models
The idea that matter consists of atoms dates back to Ancient Greek times. Over centuries, different models have tried to explain atomic structure:
- 1804 - Atoms are tiny solid spheres that can't be divided (Dalton).
- 1897 - Atoms are spheres of positive charge containing negative electrons (Thomson).
However, these models did not include a nucleus.
Rutherford's scattering experiment
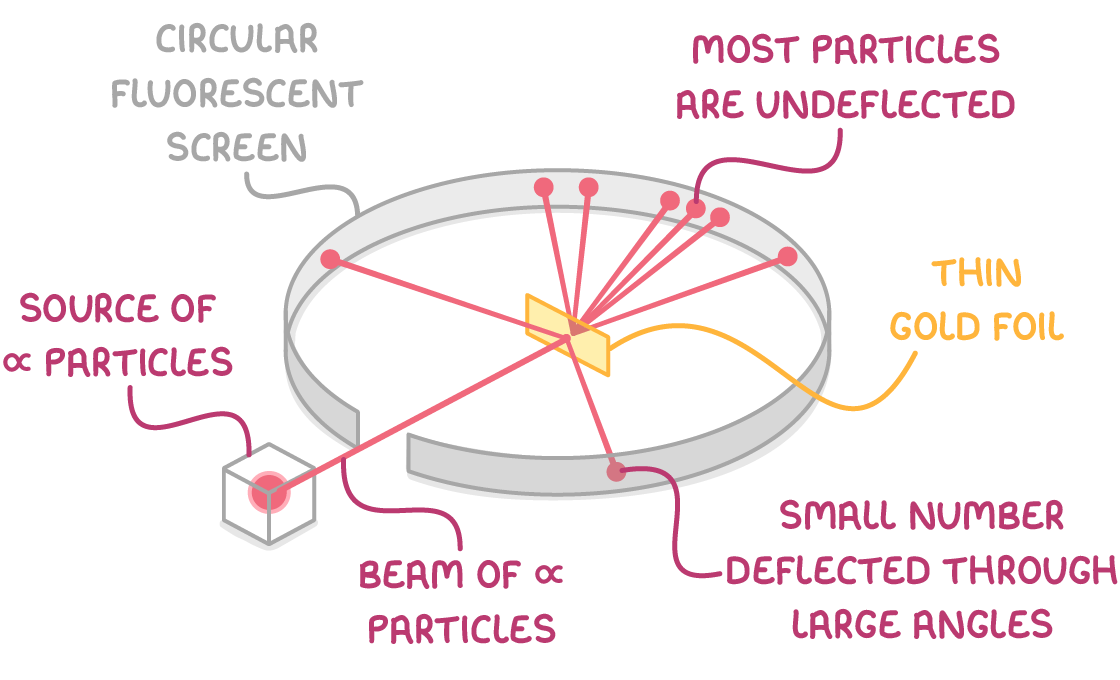
Rutherford fired alpha particles from a radioactive source at a thin gold foil. The gold foil was surrounded by a fluorescent screen which enabled the detection of the scattered alpha particles. The structure of the atom was deduced by analysis of the scattered alpha particles.
Observations made during Rutherford's experiment
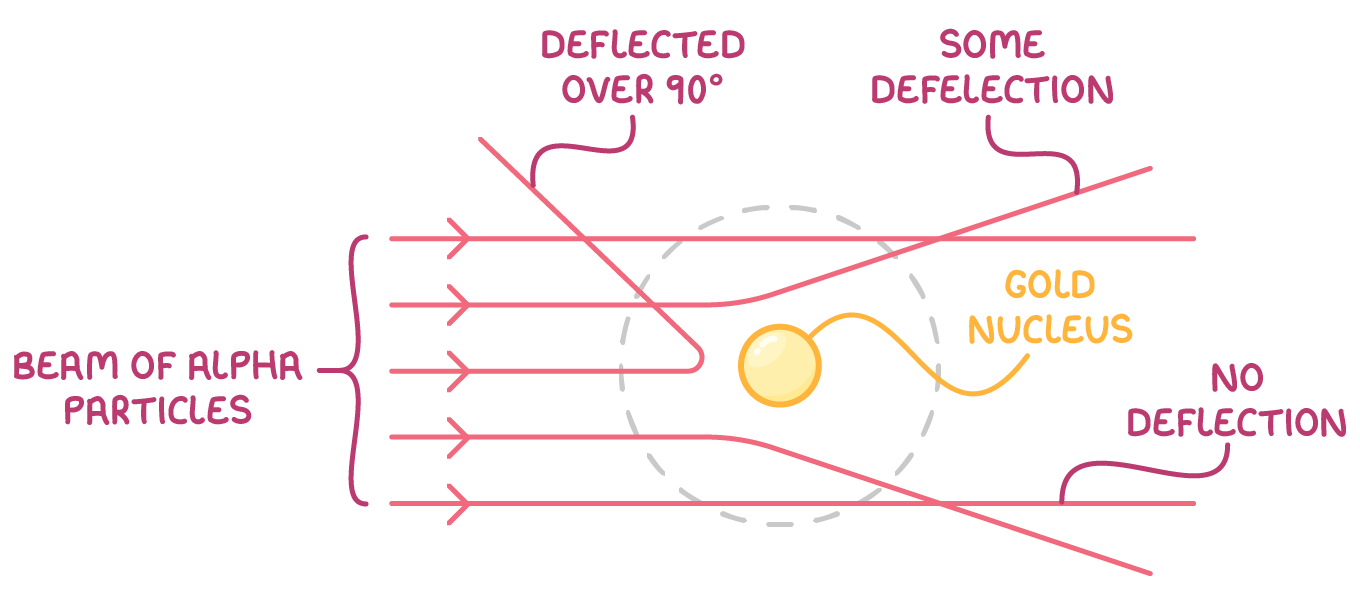
Rutherford observed:
- Most alpha particles passed through the gold foil with no deflection.
- Some alpha particles passed through the gold foil with a small deflection.
- A very small number (about 1 in 8000) alpha particles were scattered over 90°.
Conclusions made from Rutherford's results
Rutherford concluded:
- Atoms contain a small, dense, positively charged nucleus.
- The nucleus contains most of the atom's mass.
- The rest of the atom is mostly empty space.
- The nucleus has enough positive charge to repel the positively charged alpha particles.
- The nucleus must be very small to only rarely deflect alpha particles.
These discoveries overturned earlier atomic models and led to our modern understanding of atomic structure centred around a nucleus.