Changes Of State
This lesson covers:
- The three phases of matter and their particle arrangements
- Evidence for the kinetic model provided by Brownian motion
- Understanding internal energy as a combination of kinetic and potential energy
The Three Phases of Matter
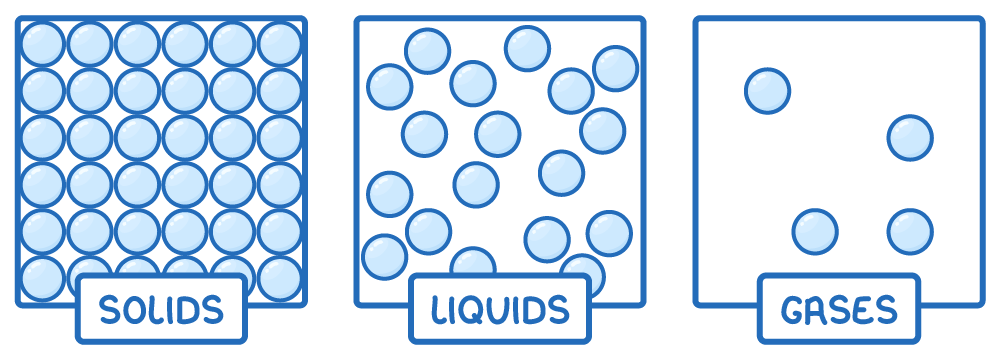
Matter exists primarily in three states: solid, liquid, and gas.
Each phase is characterised by unique particle arrangements and movements:
- Solids - Here, particles are closely packed in a regular lattice structure, vibrating slightly around fixed positions. Strong intermolecular forces keep the particles firmly in place, giving solids a definite shape and volume.
- Liquids - In liquids, particles are closer together but can move and flow around each other. This fluidity is due to weaker intermolecular forces compared to solids, allowing liquids to take the shape of their container while maintaining a consistent volume.
- Gases - Gas particles are far apart and move chaotically. They have negligible intermolecular attractions, allowing them to fill the entire volume of their container.
The kinetic model of matter explains these phases by considering particle movement and intermolecular forces.
Brownian Motion
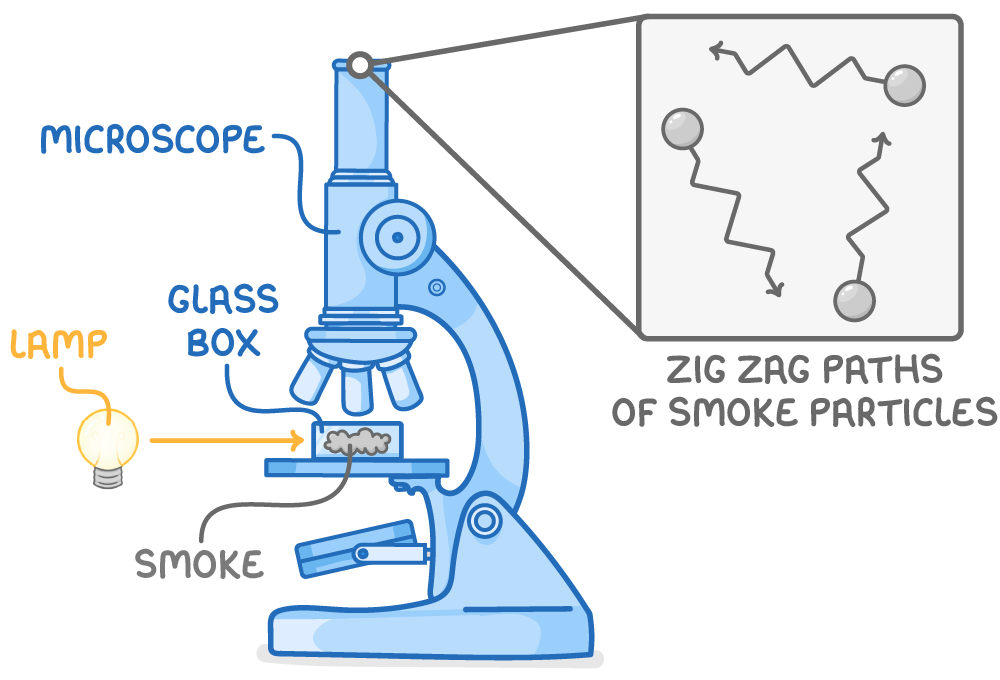
In 1827, Robert Brown discovered particles' erratic movement in fluid, a phenomenon later named Brownian motion.
This observation provided key support for the kinetic model:
- Brownian motion is observable by suspending smoke particles in air inside a jar and viewing them under a microscope.
- The random, zig-zag paths of these particles result from collisions with the fast-moving atoms and molecules of the surrounding air.
- This erratic movement is evidence of the existence of discrete, constantly moving particles, a central concept in the kinetic model of matter.
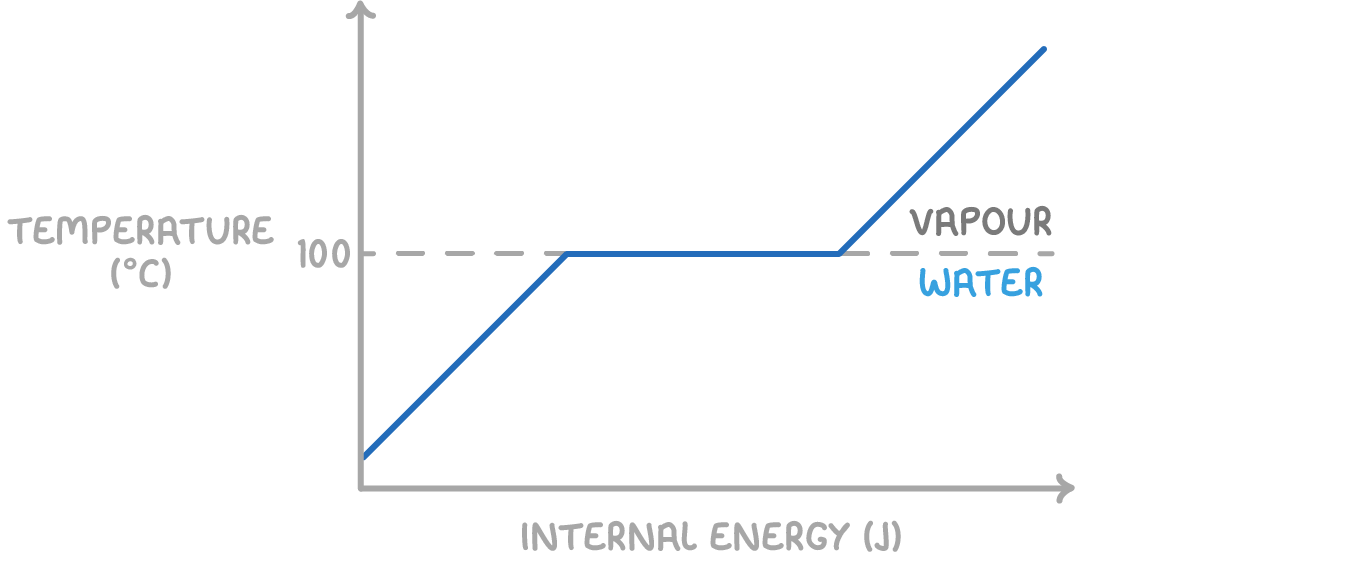
Internal energy
Internal energy refers to the total energy, both kinetic and potential, held by the particles in a system:
Internal energy = sum of kinetic energies + sum of potential energies
- Kinetic energy is linked to the particles' mass and their velocity. Higher temperatures lead to increased average speeds of particles.
- Potential energy is related to the forces of attraction between particles. Overcoming these forces, like melting ice or boiling water, requires an addition of energy.
For instance, boiling water increases its molecules' potential energy as they transition from a structured solid (ice) to a more disordered liquid and then to gas.
This change in internal energy differs from a change in temperature.