Specific Heat Capacity
This lesson covers:
- Defining specific heat capacity
- The equation linking energy, mass, specific heat capacity, and temperature change
- Methods to experimentally determine specific heat capacity
- Using the method of mixtures to estimate specific heat capacity
Specific heat capacity
Specific heat capacity (c) is the amount of energy required to raise the temperature of 1 kg of a substance by 1 K or 1°C.
Substances with a higher specific heat capacity need more energy to increase their temperature.
The relationship between energy change, mass, specific heat capacity, and temperature change is given by:
E = m c Δθ
Where:
- E = Energy change (J)
- m = Mass (kg)
- c = Specific heat capacity (J kg-1°C-1)
- Δθ = Change in temperature (K or °C)
Worked example - Calculating specific heat capacity
0.250 kg of water is heated from 12.1°C to 22.9°C using a 12 V, 5.30 A heater over 205 s. Calculate the specific heat capacity of water.
Step 1: Calculate heat energy supplied
E = V I t = 12 x 5.30 x 205 = 13,038 J
Step 2: Calculate temperature change
Δθ = 22.9 - 12.1 = 10.8 °C
Step 3: Rearranged specific heat capacity formula
c = mΔθE
Step 4: Substitution and correct evaluation
c=mΔθE=0.250×10.813,038=4,828.9 J kg−1K−1
Determining specific heat capacity experimentally
To find the specific heat capacity of a substance, you need to heat it and measure:
- The energy supplied
- The mass
- The resulting temperature change
Then use these values in the equation above to calculate c.
For a solid:

For a liquid:
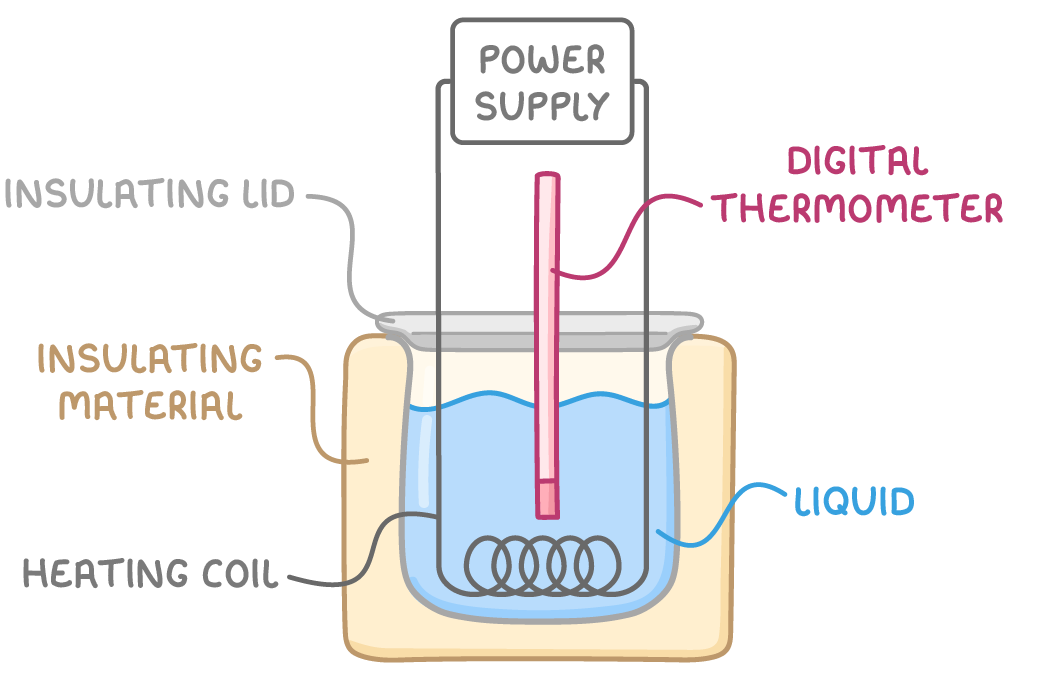
Method:
- Heat the substance, aiming for a temperature rise of about 10 K.
- Calculate the heating energy (E) using E=VIt.
- Measure the mass (m) and temperature change (Δθ).
- Substitute into E=mcΔθ to calculate c.
Estimating specific heat capacity using the method of mixtures
This method estimates a metal's specific heat capacity:
Steps:
- Heat a metal block of mass mc to T1.
- Transfer it into a calorimeter containing water of mass mw at T0.
- Measure the final steady temperature T.
- Heat gained by water = heat lost by metal block mcc(T−T0)=mwc(T1−T)
- Rearrange to find c: c=mc(T−T0)mwc(T1−T)
Worked Example - Calculating specific heat capacity using method of mixtures
A metal block of mass 0.5 kg is heated to 100°C and then placed in a calorimeter containing 0.4 kg of water at 20°C. The final temperature of the mixture is 25°C. Calculate the specific heat capacity of the metal, assuming the specific heat capacity of water is 4,200 J kg-1 K-1.
Step 1: Formula for heat transfer
Heat lost by metal = Heat gained by water
mccc(T1−T)=mwcw(T−T0)
Where:
- mc = mass of the metal block (kg)
- cc = specific heat capacity of the metal (J kg-1 K-1)
- T1 = initial temperature of the metal (°C)
- T = final temperature of the mixture (°C)
- mw = mass of the water (kg)
- cw = specific heat capacity of water (J kg-1 K-1)
- T0 = initial temperature of the water (°C)
Step 2: Rearrange to find the specific heat capacity of the metal
cc=mc(T1−T)mwcw(T−T0)
Step 3: Substitution and correct evaluation
cc=0.5×(100−25)0.4×4200×(25−20)=0.5×758400×5=37.542000=1120 J kg−1K−1
Worked Example - Estimating Energy Required to Heat a Substance
Calculate the energy required to heat 0.75 kg of aluminium from 15°C to 75°C. The specific heat capacity of aluminium is 900 J kg-1 K-1.
Step 1: Specific heat capacity formula
E=mcΔθ
Step 2: Calculate temperature change
Δθ=75−15=60∘C
Step 3: Substitution and correct evaluation
E=0.75×900×60=40,500 J
Worked example - Determining final temperature in a calorimetry experiment
0.3 kg of iron at 150°C is placed in 0.2 kg of water at 25°C in a calorimeter. The specific heat capacity of iron is 450 J/kg·K, and that of water is 4,200 J/kg·K. Calculate the final temperature of the mixture.
Step 1: Formula for heat transfer in a calorimetry experiment
Heat lost by iron = Heat gained by water
mironciron(Tinitial(iron) - T) = mwatercwater(T - Tinitial water)
Step 2: Rearrange to find final temperature (T_final)
0.3×450×(150−T)=0.2×4200×(T−25)
Step 3: Substitution and correct evaluation
135×(150−T)=840×(T−25)
20250−135T=840T−21000
975T=41250
T=97541250≈42.3∘C