Specific Latent Heat
This lesson covers:
- Explaining specific latent heat of fusion and vaporisation
- Understanding the formula connecting energy change, mass, and specific latent heat
- Recognising typical values for specific latent heat of fusion and vaporisation
- Describing experimental methods for determining specific latent heat
Specific Latent Heat
Specific latent heat is the amount of energy required to change the state of one kilogram of a substance without changing its temperature.
There are two main types:
- Specific Latent Heat of Fusion - This is the energy needed to change a substance from solid to liquid without changing its temperature.
- Specific Latent Heat of Vaporisation - This is the energy needed to change a substance from liquid to gas without changing its temperature.
Some typical values for these are:
- Water (fusion): 334 kJ kg-1
- Water (vaporisation): 2,260 kJ kg-1
- Ethanol (vaporisation): 840 kJ kg-1
Relationship between energy change, mass and specific latent heat
The energy change is directly proportional to the mass of the substance and its specific latent heat value.
The energy change (E) required for a state change is calculated using the formula:
E = m L
Where:
- E = energy change in joules (J)
- m = mass of the substance in kilograms (kg)
- L = specific latent heat in joules per kilogram (J kg-1)
Worked Example - Calculating the energy required to melt ice
Calculate the energy required to melt 0.5 kg of ice. The specific latent heat of fusion for water is 334 kJ kg-1.
Step 1: Formula
E = m L
Step 2: Substitution and correct evaluation
E = 0.5 x 334,000 = 167,000 J
Determining specific latent heat experimentally
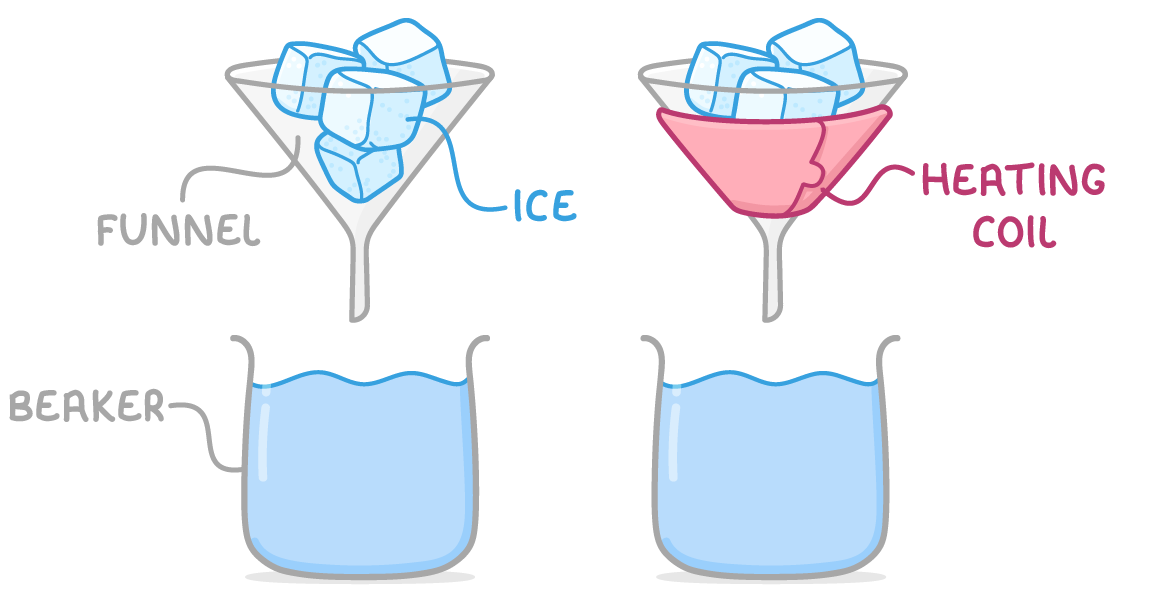
Experimentally, specific latent heat is determined by careful measurement of energy input and mass change during the state change of a substance.
To determine the specific latent heat of fusion of ice:
- Place equal masses of ice in two funnels above beakers.
- Turn on the heater around one funnel only and supply a measured quantity of energy for a set time, such as 3 minutes.
- Measure the mass of water collected in both beakers.
- Find the difference in mass to negate the effect of ambient heat.
- Calculate the specific latent heat of fusion by dividing the energy supplied by the mass of ice that melted.
To determine the specific latent heat of vaporisation of water:
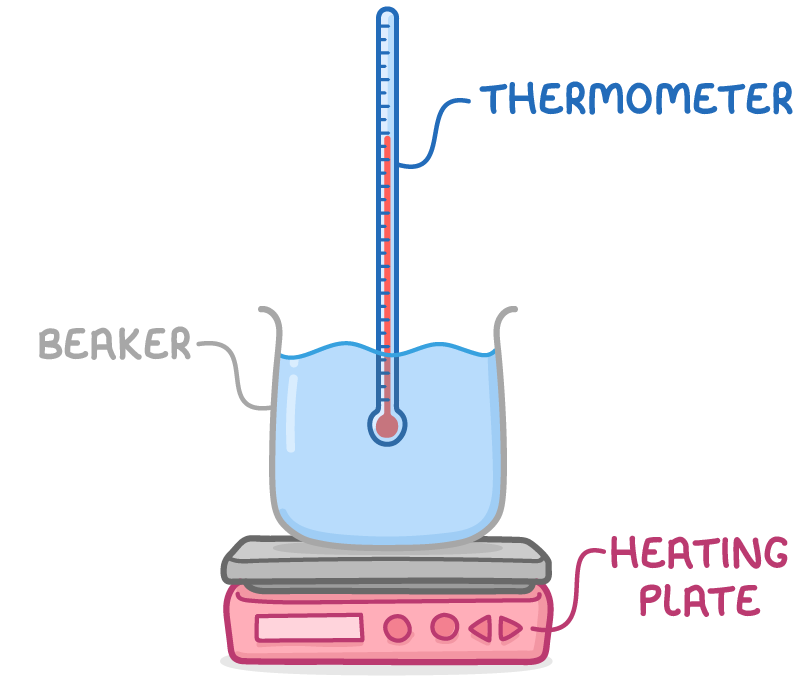
- Place a known mass of water in a beaker on a heating plate.
- Supply a measured quantity of energy until the water reaches boiling point.
- Allow the water to boil for a set period, like 3 minutes.
- Measure the remaining water mass.
- Calculate the specific latent heat of vaporisation by dividing the energy supplied by the mass of water that vaporised.
Worked example - Calculating the energy required to vaporise water
Now, let's calculate the energy needed to vaporise 2 kg of water.
The specific latent heat of vaporisation for water is 2,260 kJ kg-1.
Step 1: Formula
E = m L
Step 2: Substitution and correct evaluation
E = 2 x 2,260,000 = 4,520,000 J