Nuclear Fission
This lesson covers:
- Understanding the concept of heavy unstable nuclei splitting into smaller nuclei, known as nuclear fission
- Exploring how energy is released during fission
- The reasons behind the instability of large nuclei leading to spontaneous fission
- The limitations this instability imposes on the number of possible elements
Nuclear fission
Certain heavy and unstable nuclei, such as uranium, can spontaneously split into two smaller nuclei, a process referred to as nuclear fission. This process typically results in the formation of smaller, more stable nuclei, along with the release of a few neutrons.
There are two types of fission:
- Spontaneous fission - occurs without any external intervention
- Induced fission - initiated by absorbing an external neutron into the nucleus
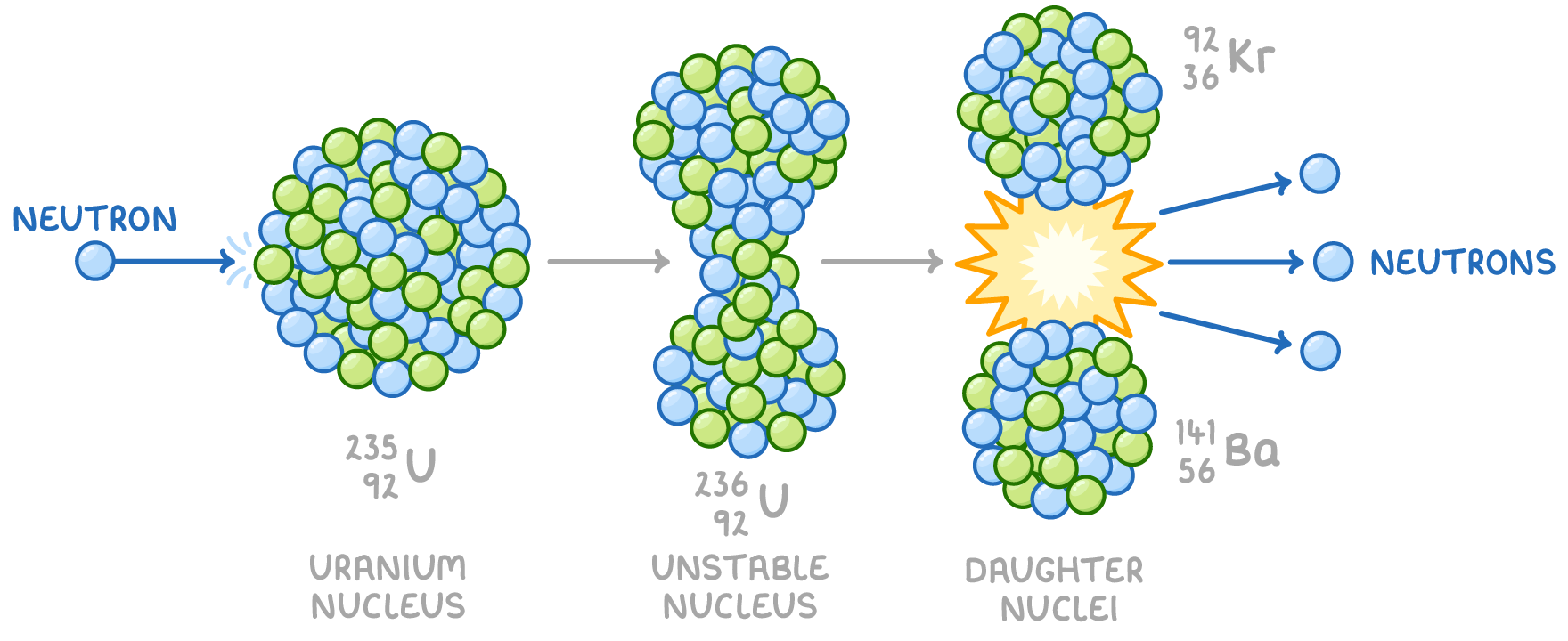
For instance, uranium-235 becomes highly unstable and splits after absorbing a low-energy neutron, known as a thermal neutron.
Nuclear fission releases energy
The energy release during fission reactions is significant because the resulting daughter nuclei have:
- A higher binding energy per nucleon, indicating greater stability
- A reduced overall mass
The difference in mass is converted into energy, following Einstein's equation (E = mc2).
The instability of large nuclei
As nuclei grow in size, they become more unstable. The vast and complex structures of giant nuclei make them prone to spontaneous vibrations and distorted shapes, which further contribute to their instability.
- Giant nuclei are more likely to undergo spontaneous fission.
- An increase in nucleon numbers exacerbates instability.
This inherent instability naturally limits the maximum number of protons and neutrons that can coexist within a nucleus, effectively capping the number of elements that can exist.