Nuclear Instability
This lesson covers:
- The reasons behind nuclear instability
- The various forms of radioactive decay in unstable nuclei
- The application of a stability graph to discern patterns of nuclear stability and preferred decay modes
What causes nuclear instability?
The nucleus of an atom is composed of protons and neutrons, which are tightly bound together by a strong nuclear force. Despite this, the electromagnetic force causes repulsion between the protons, attempting to tear the nucleus apart. Stability in the nucleus is a delicate balance, and it can be disrupted, leading to instability when:
- The neutron count is excessively high,
- The neutron count is insufficient,
- The total count of nucleons (protons and neutrons) becomes overly large, rendering the nucleus too hefty,
- An overabundance of energy is present within the nucleus.
Modes of radioactive decay
To achieve a stable state, unstable nuclei may undergo several types of spontaneous radioactive decay processes:
- β- decay - involves a neutron transforming into a proton, during which an electron and an antineutrino are emitted.
- β+ decay -sees a proton converting into a neutron, with the emission of a positron and a neutrino.
- α decay - entails the emission of an α particle, which is essentially a helium nucleus.
These decay processes help to lower the nucleus's energy, adjust the number of nucleons, and modify the neutron-to-proton ratio towards a more stable configuration.
Nuclear stability graph
A nuclear stability graph visually represents the relationship between the number of protons (Z) and the number of neutrons (N) within nuclei.
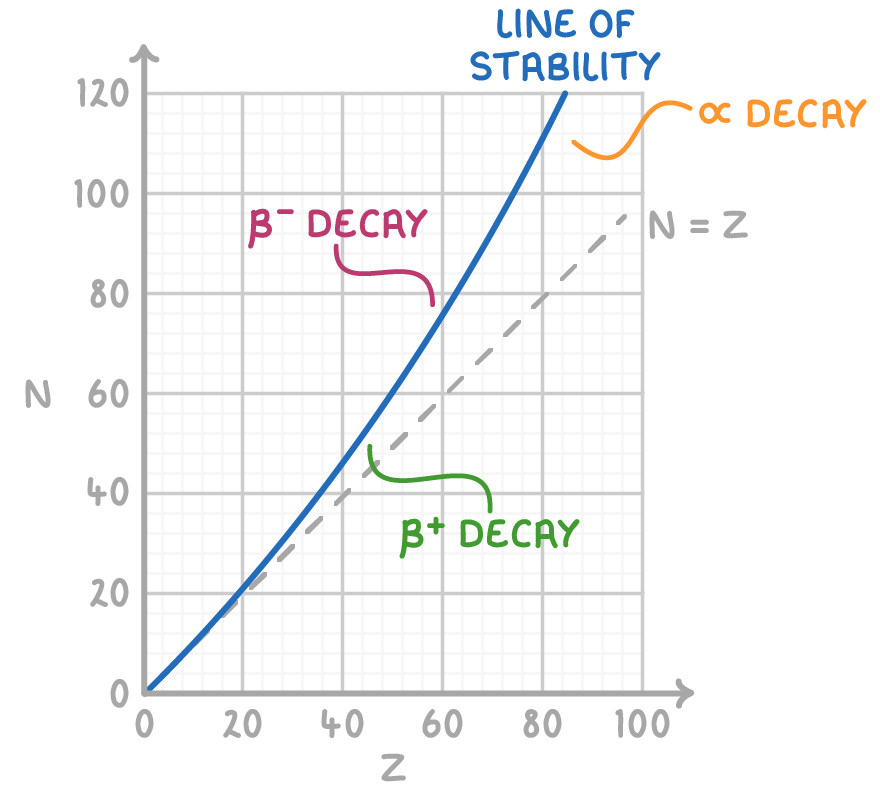
Interpreting the graph:
- Nuclei that are stable are found along the line of stability.
- Nuclei positioned above the line possess too many neutrons and typically undergo β- decay to shed the excess.
- Nuclei located below the line have too many protons and are prone to β+ decay in order to correct the imbalance.
- Heavier nuclei, situated to the right of the graph, often decay through α emission to reduce their mass.