Constituents Of The Atom
This lesson covers:
- The particles that make up an atom (protons, neutrons, electrons)
- The charge and mass of these particles
- Nuclide notation
- Isotope notation
Inside the atom

An atom consists of three types of subatomic particle:
- Protons
- Neutrons
- Electrons
Protons and neutrons make up the central core of an atom called the nucleus. The electrons orbit around the nucleus.
Most of an atom is empty space as the electrons orbit the nucleus at relatively large distances.
Particles have different masses and charges
The particles in an atom have different masses and charges:
| Relative Charge | Absolute Charge (C) | Relative mass | Mass (kg) | |
|---|---|---|---|---|
| Proton | + 1 | + 1.6 x | 1 | 1.67 x 10 |
| Neutron | 0 | 0 | 1 | 1.67 x 10 |
| Electron | - 1 | - 1.6 x 10 | 0.0005 | 9.11 x 10 |
The absolute charges and masses are tiny values so to make them easier to work with, relative charge and mass values are sometimes used.
Key numbers describing an atom
Two key numbers are used to describe an atom:
- Proton number (Z) - The number of protons in the nucleus
- Nucleon number (A) - The total number of protons and neutrons
These numbers tell you what element and isotope an atom is.
Isotopes
Atoms of the same element can have the same proton number but different nucleon numbers. These atoms are called isotopes.
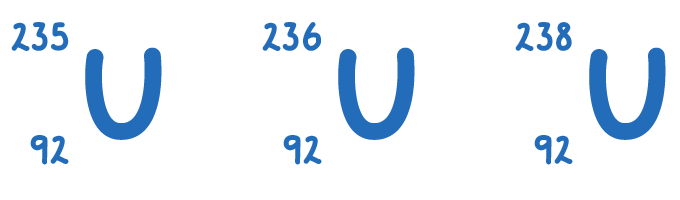
| Isotope | Atomic Number | Mass number | Number of Neutrons |
|---|---|---|---|
| U - 235 | 92 | 235 | 235 - 92 = 143 |
| U - 236 | 92 | 236 | 236 - 92 = 144 |
| U - 238 | 92 | 238 | 238 - 92 = 146 |
Here we can see each isotope of Uranium has 92 protons but has a different number of neutrons.