Radioactive Decay & Half-life
This lesson covers:
- What the terms 'activity' and 'half-life' mean
- How to interpret a graph of radioactive decay
- How to calculate the activity and half-life of a radioactive material
Which of the following describes the decay process for a single atom?
The decay occurs at regular intervals
The decay is random
|
magnitude / rate / isotopes
'Activity' is the overall of decay of all of the radioactive in our sample.
|
We measure radioactive activity as the average number of decays per second.
What are the units of activity?
Becquerels (Bq)
Webers (Wb)
Teslas (T)
Volts (V)
|
Which of the following are definitions for the 'half-life' of a radioactive sample?
(Select all that apply)
The time taken for the activity to half
The time taken for the number of radioactive nuclei in a sample to halve
The time taken for the nuclei to lose half their electrons
|
True or false? As a radioactive sample decays over time, its half life decreases.
True
False
|
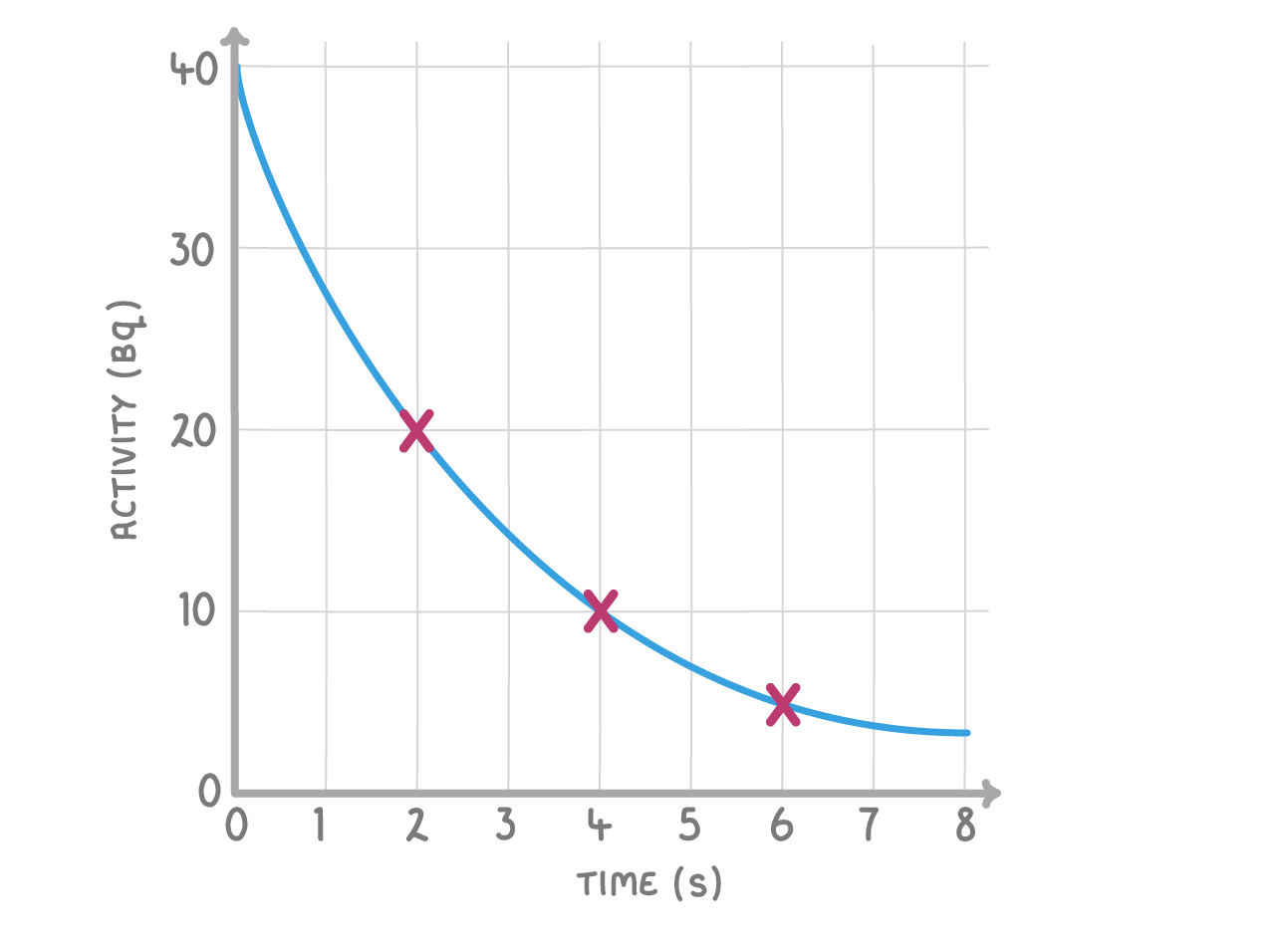
The above graph shows a radioactive decay curve.
What is the half-life of the material?
s
|
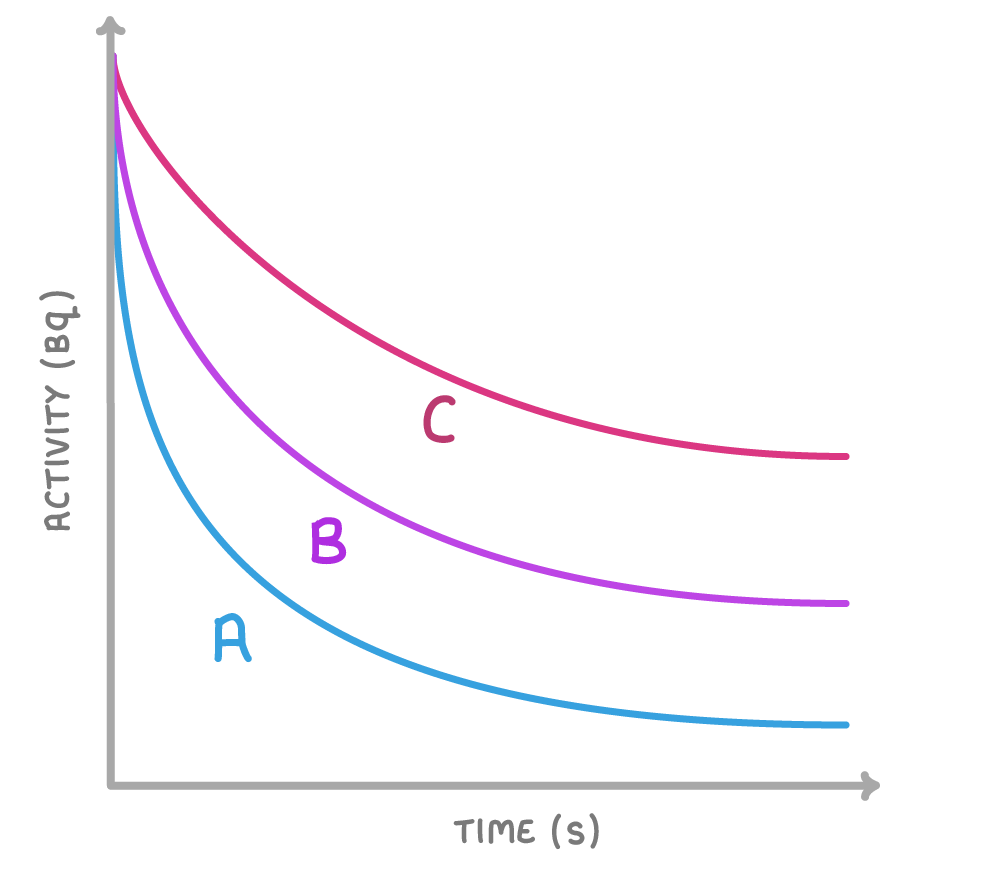
The above graph shows three different radioactive materials.
Which graph shows the radioactive material with the shortest half-life?
A
B
C
|
What device is used to record radioactive decays?
Geiger-Muller tube and counter
Voltmeter
Galvanometer
Oscilloscope
|
The activity of a radioactive material started at 240 Bq and is now 30 Bq.
How many half-lives have elapsed?
half-lives
|
The activity of a radioactive material is 800 Bq and its half-life is 5 hours.
What is the activity of the radioactive material after 15 hours?
Bq
|
A radioactive isotope has a half-life of 10 minutes.
If the activity was initially 800 Bq, calculate how long it would take for the activity to fall to 50 Bq.
min
|