Density
This lesson covers:
- What 'density' is
- The density equation:
- How to find the density of an unknown substance experimentally
What is the formula for density?
|
weight / mass / volume / size
Density is a measure of how much a substance has, per unit of its .
|
What are the units of density?
kg m3
kg/m3
m3/kg
kg m/s
|
What is the symbol formula for density?
|
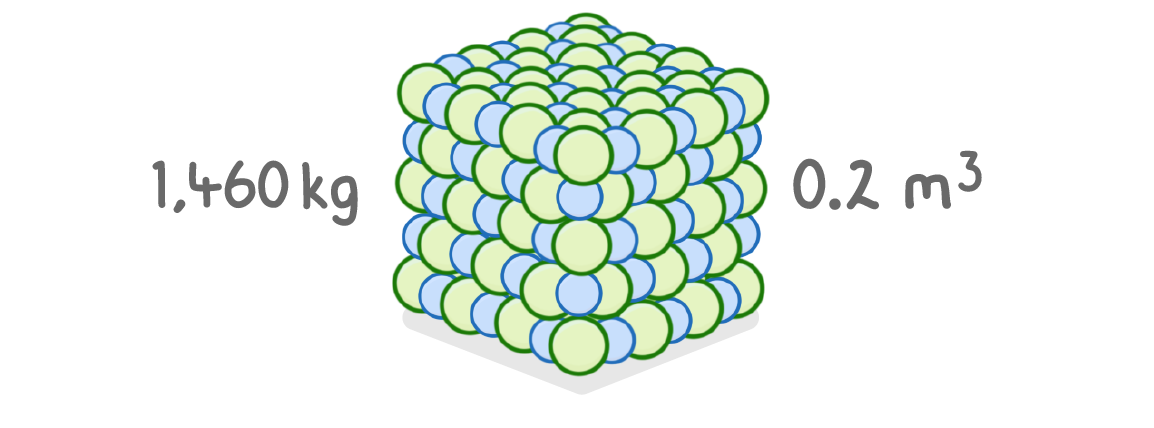
A substance has a mass of 1,460 kg and a volume of 0.2 m3.
What is the density of the substance?
kg/m3
|
How to calculate the density of a solid experimentally
watch / balance / mass / area / volume / Eureka can
- Measure the mass of the solid using a .
- If the shape is regular, measure the of the solid using geometry.
- If the shape is irregular, measure the volume of the solid by adding it to a filled with water. This will cause a volume of water exactly equal to the volume of the solid to flow into the measuring cylinder.
- Use the formula to calculate the density of the solid from the and volume measurements.
|
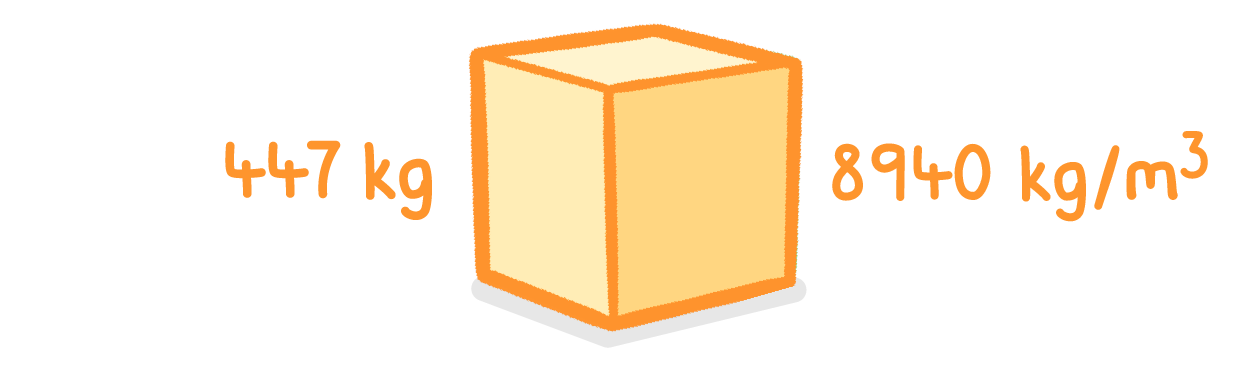
A 447 kg block of copper has a density of 8940 kg/m3.
What is the volume of this block of copper?
m3
|
The most common units of density are g/cm3 and kg/m3.
To convert g/cm3 to kg/m3 you have to:
Multiply the g/cm3 value by 1000
Divide the g/cm3 value by 1000
|
The density of sodium is 0.968 g/cm3.
What is its density in kg/m3?
968 kg/m3
0.000968 kg/m3
|
How to calculate the density of a liquid experimentally:
density / balance / colour / mass / volume
- Place an empty measuring cylinder onto a , then 'zero' the reading.
- Add some of the liquid to the measuring cylinder.
- Read the of the liquid from the measuring cylinder.
- Read the of the liquid from the balance.
- Use the formula to calculate the density from the mass and volume measurements.
|
Which of these methods are used to reduce the uncertainty in experimental measurements of density?
(Select all that apply)
Take multiple measurements and calculate the mean
When measuring the density of liquids, use a larger volume of liquid
Take multiple measurements and use the largest value
When measuring the density of liquids, use a smaller volume of liquid
|