Factors Affect Gas Pressure
This lesson covers:
- How gas particles behave, and how that behaviour creates pressure
- How temperature, concentration, and volume affect pressure
- How temperature and concentration affect volume in a flexible container
True or false? Gas particles in a container move around randomly, bouncing off other particles and the walls of the container.
True
False
|
What is the formula for pressure?
|
Which factors can increase the pressure of a gas?
(Select all that apply)
More collisions with the walls of the container
Faster moving particles
Slower moving particles
Fewer collisions with the walls of the container
|
How volume affects pressure in a gas
less / more / area / volume / increase / decrease
- Decreasing the volume of the container, whilst keeping the number of gas particles the same, will the concentration.
- In this smaller volume, collisions between particles of gas and the walls of the container will be frequent.
- A greater number of collisions per unit of wall means the pressure increases.
|
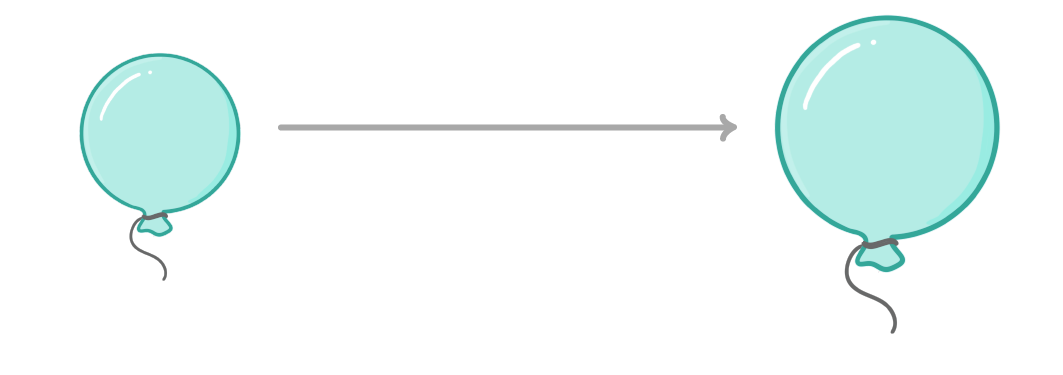
How temperature and concentration affect a flexible container
force / pressure / volume / expand / contract
- Some containers, such as balloons, are flexible.
- An increase in on the walls of the container would just cause the container to .
- Therefore, changing temperature or concentration will change the of the container, rather than the of the gases inside.
|
Which of the following statements are true?
(Select all that apply)
Increasing concentration increases pressure (in a flexible container)
Increasing temperature increases pressure (in a fixed volume container)
Increasing concentration increases pressure (in a fixed volume container)
Increasing temperature increases volume (in a flexible container)
|