Evaporation
This lesson covers:
- The difference between evaporation and boiling
- The effect of temperature, surface area, and air flow on the rate of evaporation
- The cooling effect of evaporation
Evaporation vs Boiling People often think 'boiling' and 'evaporating' are the same thing. However, in science they refer to different processes. |
Evaporation 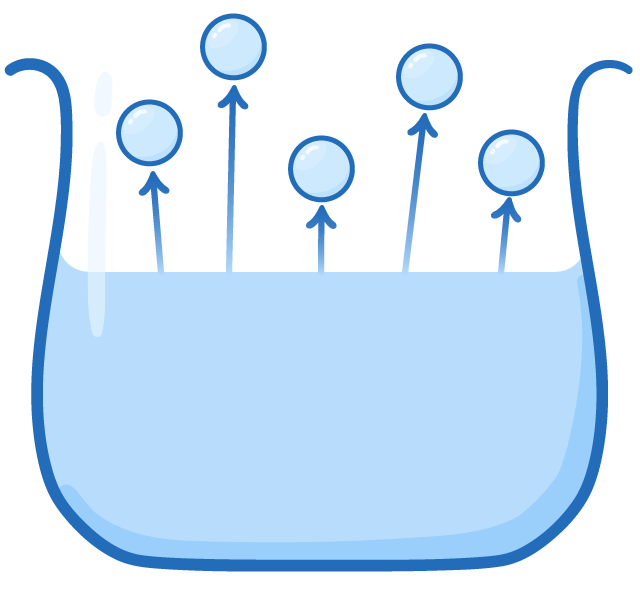
|
Boiling 
|
Factors affecting evaporation rate The rate of evaporation is affected by several factors including temperature, surface area, and air flow. |
Temperature
|
Surface area
|
Air flow
|
Evaporation lowers the temperature of a liquid
- Molecules that escape the surface of a liquid are usually those with the most kinetic energy.
- This leaves the remainder of the liquid with a lower kinetic energy on average.
- As the average kinetic energy decreases, the temperature of the liquid decreases.
Evaporation occurs when liquid molecules escape from the surface of a liquid and become gas. At what temperature does evaporation occur?
At any temperature
Above the boiling point
At the boiling point
Below the boiling point
|
Which of the following are true about boiling?
Boiling only happens once the boiling point has been reached.
Boiling is when bubbles of gas form within the liquid.
Boiling occurs when liquid molecules escape from the surface of a liquid and become gas.
Boiling occurs at any temperature.
|
Which of the following factors affect the rate of evaporation?
Surface area
Air flow
All of the above
Temperature
|
How does temperature affect the rate of evaporation?
As temperature increases, the rate of evaporation increases.
As temperature increases, the rate of evaporation stays the same.
Temperature has no effect on the rate of evaporation.
As temperature increases, the rate of evaporation decreases.
|
How does surface area affect the rate of evaporation?
Surface area has no effect on the rate of evaporation.
A larger surface area decreases the rate of evaporation.
A larger surface area has no effect on the rate of evaporation.
A larger surface area increases the rate of evaporation.
|
How does air flow affect the rate of evaporation?
Moving air causes boiling instead of evaporation.
Air flow has no effect on the rate of evaporation.
Moving air increases the rate of evaporation.
Moving air decreases the rate of evaporation.
|