Particle movement
This lesson covers:
- Brownian motion
- Diffusion
- The effect of temperature on particle movement
Brownian Motion
Brownian motion describes the random, zigzag movement of tiny particles when suspended in a liquid or gas.
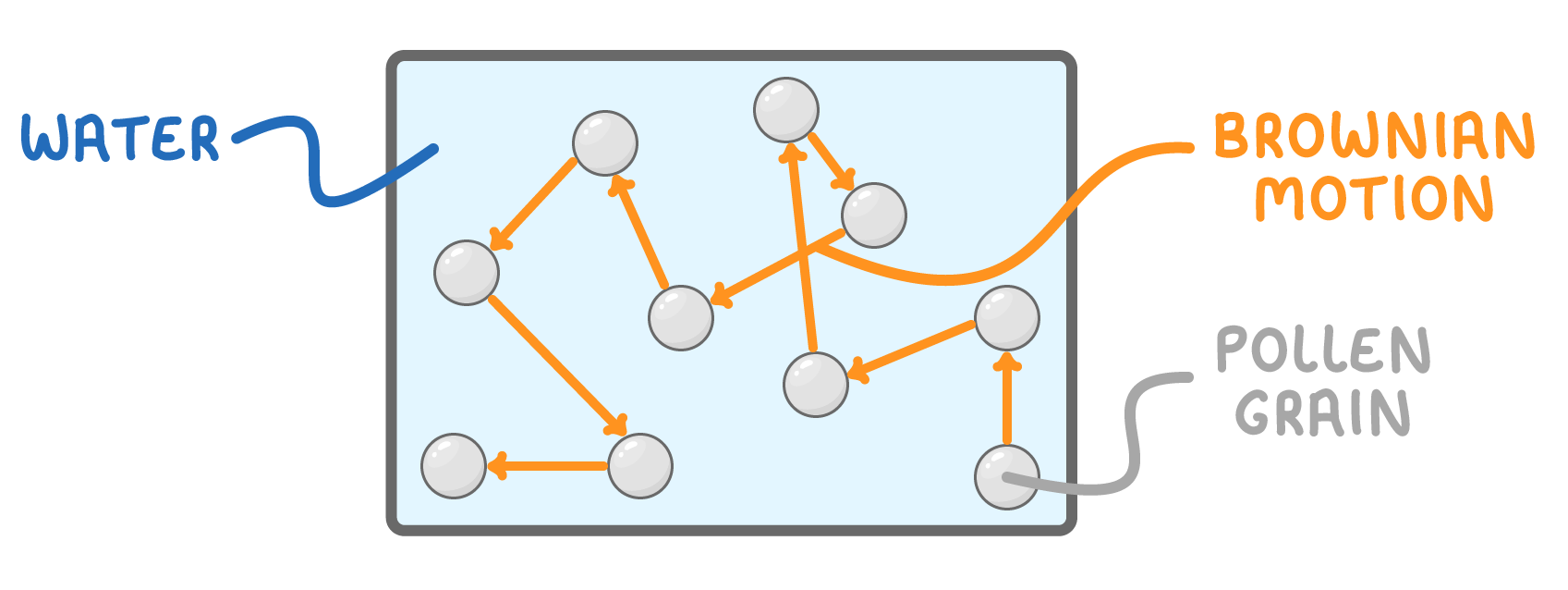
- In 1827, scientist Robert Brown observed the irregular motion of pollen particles suspended in water under a microscope.
- This type of random particle movement was later termed Brownian motion.
- Brownian motion involves lighter particles like air molecules colliding with heavier particles like smoke, causing the heavier particles to move around randomly.
Random motion of particles causes diffusion
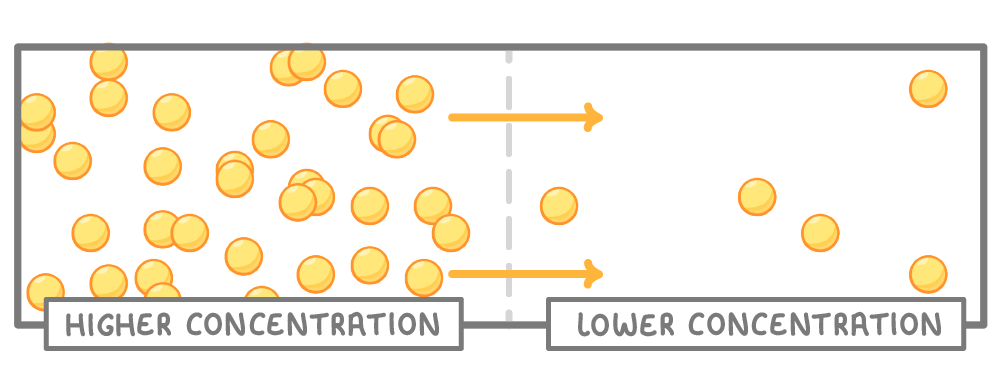
- Particles randomly move around and collide.
- This causes them to spread from areas of high concentration to areas of low concentration.
- This continues until particles are evenly dispersed throughout.
Increasing temperature, increases particle movement

- At higher temperatures, particles have more kinetic energy so move quicker and collide more forcefully.
- With faster, more energetic motion, particles require more space between each other. This causes expansion of the material.
- There is increased particle collision with container walls, increasing the pressure.