Preparation of Aliphatic & Aromatic Amines
This lesson covers:
- How aliphatic amines are produced
- The reaction mechanism involved in producing aliphatic amines
- How aromatic amines are produced
Reacting halogenoalkanes with ammonia and amines
Aliphatic amines can be produced by reacting a halogenoalkane with ammonia, primary amines, or secondary amines in a nucleophilic substitution reaction.
- Reaction with ammonia to form primary amines:
Primary aliphatic amines can be produced by heating a halogenoalkane with excess ethanolic ammonia.
For example, bromoethane reacts with ammonia to give ethylamine:
CH3CH2Br + 2NH3 ➔ CH3CH2NH2 + NH4Br
- Reaction with primary amines to form secondary amines:
Secondary aliphatic amines can be produced by reacting a halogenoalkane with a primary amine.
For example, bromoethane reacts with ethylamine to give diethylamine:
CH3CH2NH2 + CH3CH2Br ➔ (CH3CH2NH2)2NH + HBr
- Reaction with secondary amines to form tertiary amines:
Tertiary aliphatic amines can be produced by reacting a halogenoalkane with a secondary amine.
For example, bromoethane reacts with diethylamine to give triethylamine:
(CH3CH2)2NH + CH3CH2Br ➔ (CH3CH2)3N + HBr
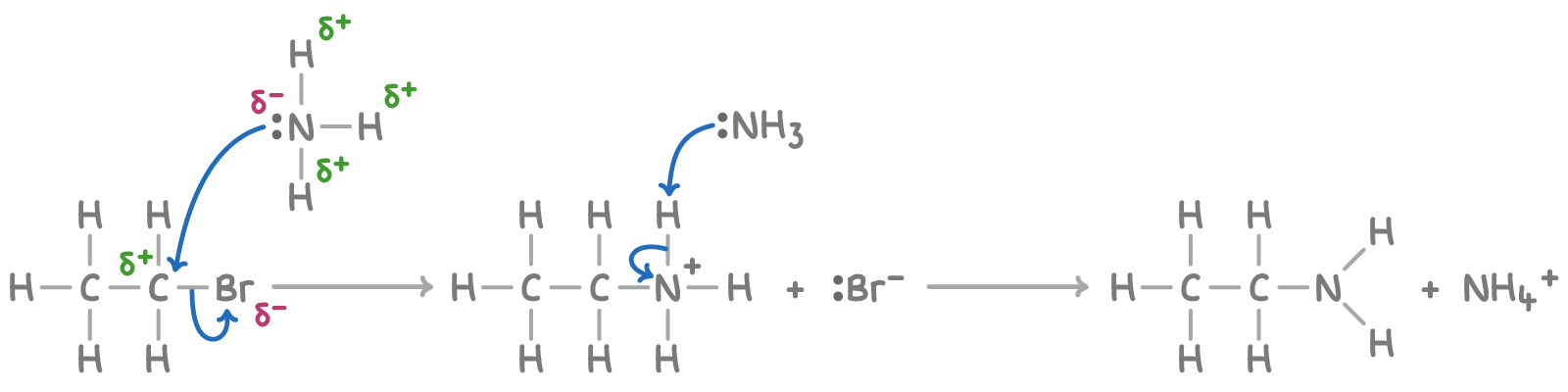
Mechanism of amine formation
The nucleophilic substitution mechanism for these reactions involves two steps:
- The nucleophile (ammonia or amine) attacks the halogenoalkane, displacing the halogen and forming an alkylammonium salt.
- The alkylammonium salt is then deprotonated by a base (e.g., the nucleophile or a separate base) to form the amine product.
For example, the mechanism for the reaction between bromoethane and ammonia is:
Aromatic amines from nitro compounds
Aromatic amines are produced by reducing nitro compounds in a two-step process:
- The nitro compound is heated under reflux with tin and concentrated HCl to form an ammonium salt.
- The ammonium salt is then treated with aqueous NaOH to give the free amine.
For example, nitrobenzene is reduced to phenylamine via phenylammonium chloride:

Aromatic amines like this are useful in organic synthesis for making pharmaceuticals, dyes, and other compounds.