Practical Techniques in Organic Chemistry
This lesson covers:
- Refluxing reactions
- Distillation
- Purification techniques
Refluxing reactions
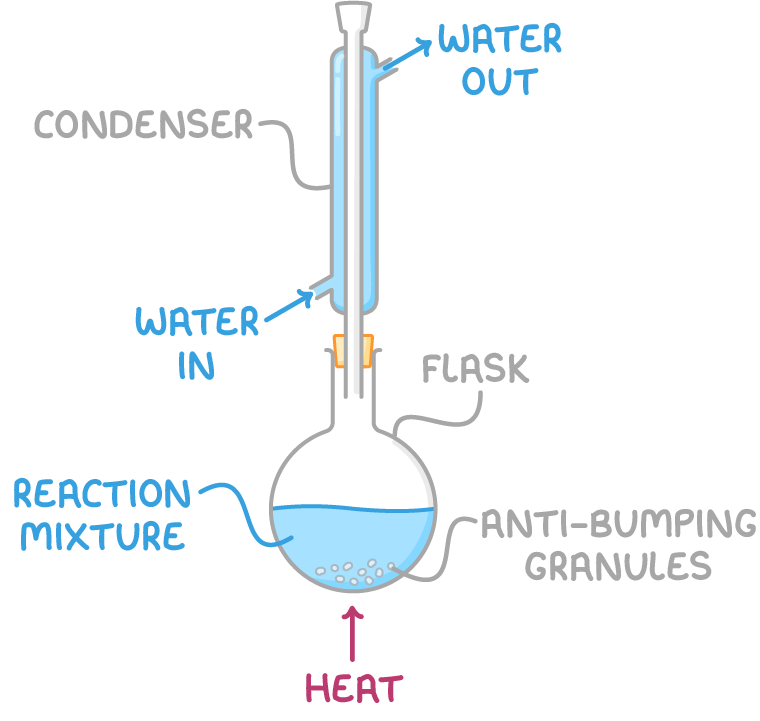
Refluxing is a technique used to heat reactions involving volatile, flammable organic compounds:
- Organic reactions proceed slowly, and many organic substances have low boiling points. Heating in an open vessel causes evaporation before reactions can take place.
- Refluxing involves heating the reaction mixture in a round-bottomed flask equipped with a condenser.
- Anti-bumping granules are added to the flask to prevent violent boiling.
- The condenser has cold water flowing through it, which condenses evaporated organic vapours back into liquid, returning them to the reaction vessel.
- This process allows the organic substances to be heated to their boiling point, speeding up the reaction, without losing material.
- Heating is done electrically to avoid open flames that could ignite flammable vapours.
Distillation
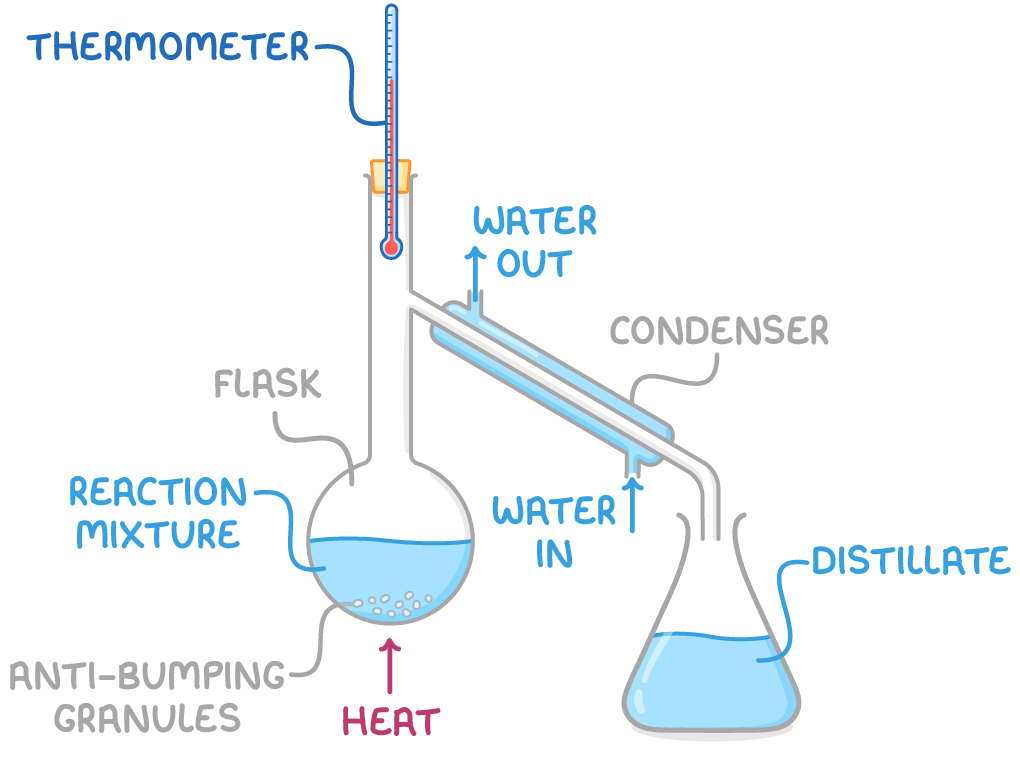
Distillation is used to separate mixtures based on differences in boiling point:
- The mixture is heated gently in a distillation apparatus.
- Components evaporate in order of their increasing boiling points.
- A thermometer is used to monitor the boiling point of the distilling vapour. This helps to identify the substance evaporating.
- If the boiling point of the desired product is known, collection can commence when the thermometer shows this temperature.
- In some reactions, distillation is employed to separate the product from the reaction mixture when the product has a lower boiling point than the reactants.
- As the reaction progresses, the product evaporates from the mixture and is collected separately through condensation, preventing it from undergoing further reaction with the remaining reactants.
Techniques to purify an organic liquid
Additional methods can remove impurities from an organic liquid:
- Redistillation - Repeated distillation helps to separate the pure volatile liquid product from impurities with different boiling points. The pure product distils over at its characteristic boiling point, leaving impurities behind.
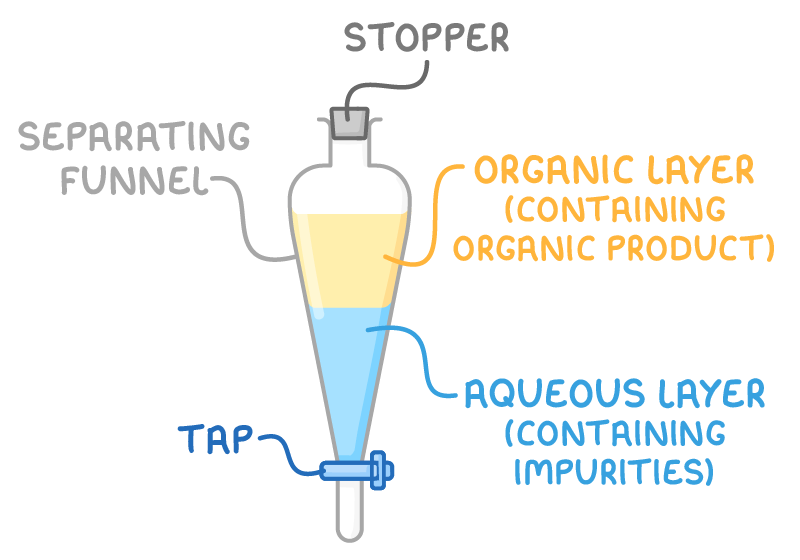
- Extraction - When water is added to a mixture of the organic liquid and an organic solvent in a separating funnel, two immiscible liquids form. The organic liquid product dissolves in the organic layer, while water-soluble impurities dissolve in the aqueous layer. The layers are then drained off separately from the tap at the bottom of the funnel.
3. Drying - Traces of water dissolved in organic products can be removed using drying agents such as anhydrous magnesium sulfate or anhydrous calcium chloride.
4. Filtration - After drying, the solid drying agent is removed by filtering the mixture, leaving behind the dry purified organic product.