Directing Groups
This lesson covers:
- What a directing effect means
- How electron density affects substitution position
- The directing effects of electron donating and withdrawing groups
- Predicting products based on substituent effects
Introduction to directing effects
In organic chemistry, a directing effect refers to the influence of a functional group on a benzene ring on the position where a second substituent is added during an electrophilic aromatic substitution reaction. Directing groups are crucial in controlling the position of the newly added substituent, ensuring that the desired product is obtained.
In an unsubstituted benzene ring, all six carbons are chemically equivalent due to the delocalised π-electron system, meaning an electrophile could react with any carbon equally. However, introducing a functional group alters the electron density at specific carbons, making them more or less reactive towards electrophiles.
The nature of the functional group, whether electron-donating or electron-withdrawing, determines which positions on the ring become activated or deactivated for electrophilic substitution.
Electron donating groups direct substitution to carbons 2-, 4- and 6-
Electron-donating groups, such as -OH and -NH2, contribute electrons into the delocalised π-system of the ring, increasing its electron density. Specifically, they enhance the electron density at carbons 2-, 4-, and 6-, making these positions more likely to react with electrophiles.

Thus, electron donating groups activate positions 2-, 4-, and 6- for electrophilic attack.
Electron withdrawing groups direct substitution to carbons 3- and 5-
Electron-withdrawing groups like -NO2 do not have orbitals that overlap with the ring's π-system. Instead, due to their electronegativity, they pull electron density away from the ring, particularly from positions 2-, 4-, and 6-.
This action directs electrophilic substitution towards the 3- and 5- positions, which retain relatively more electron density.
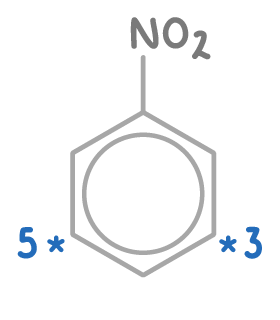
Thus the -NO2 group activates positions 3- and 5- for electrophilic attack.
Predicting substitution products
By identifying whether a substituent is electron donating or withdrawing, you can determine which positions will be activated or deactivated.
This enables you to predict the major product of electrophilic aromatic substitution reactions by following these rules:
- Electron-donating groups direct substitution to carbons 2-, 4-, and 6-.
- Electron-withdrawing groups direct substitution to carbons 3- and 5-.
The table below summarises the positions activated by different substituents on a benzene ring.
| Electron-donating groups that direct substitution to carbons 2-, 4-, and 6- | Electron-withdrawing groups that direct substitution to carbons 3-, and 5- |
|---|---|
| –NH2 | –NO2 |
| –OH |
Worked example 1 - Predicting substitution products
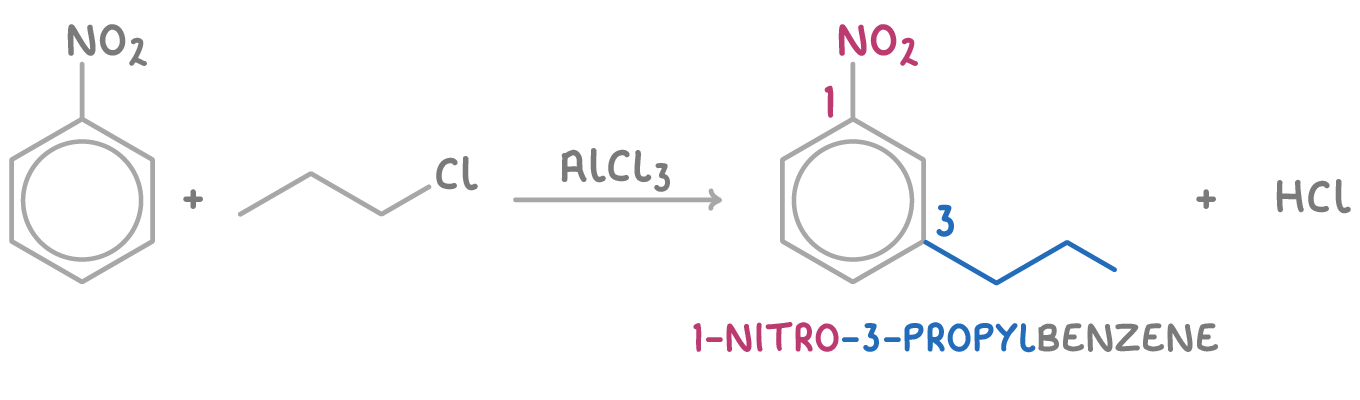
Predict the major product when nitrobenzene reacts with propyl chloride in the presence of aluminium chloride:
Step 1: Analysis
The nitro group (-NO2) is an electron withdrawing group, deactivating positions 2, 4, and 6.
This leads to the propyl substituent being directed to the relatively more activated 3 position.
Step 2: Prediction
The major product will be 1-nitro-3-propylbenzene.