Brønsted-Lowry Acids and Bases
This lesson covers:
- The Brønsted-Lowry definitions of acids and bases
- Monobasic, dibasic, and tribasic acids
- Conjugate acid-base pairs
- Reactions of acids with metals and bases
- The development of acid-base theories over time
- The ionic product of water, Kw
Brønsted-Lowry acids are proton donors, bases are proton acceptors
According to the Brønsted-Lowry theory:
- An acid is defined as a substance that donates a proton (H+).
- A base is defined as a substance that accepts a proton.
For instance, when a Brønsted-Lowry acid (HA) is dissolved in water, it donates a proton to a water molecule, forming a hydronium ion (H3O+):
HA(aq) + H2O(l) ⇌ H3O+(aq) + A-(aq)
Conversely, when a Brønsted-Lowry base (B) is mixed with water, it accepts a proton from a water molecule, forming a hydroxide ion (OH-):
B(aq) + H2O(l) ⇌ BH+(aq) + OH-(aq)
Some acids can donate more than one proton
Acids are categorised based on their capacity to donate protons:
- Monoprotic acids - These acids are capable of donating one proton per molecule, for example, hydrochloric acid (HCl) and nitric acid (HNO3):
HCl(aq) ➔ H+(aq) + Cl-(aq)
HNO3(aq) ➔ H+(aq) + NO3-(aq)
- Diprotic acids - These acids can donate two protons per molecule, for example, sulfuric acid (H2SO4):
H2SO4(aq) ➔ 2H+(aq) + SO42-(aq)
- Triprotic acids - These acids are able to donate three protons per molecule, for example, phosphoric acid (H3PO4):
H3PO4(aq) ➔ 3H+(aq) + PO43-(aq)
Acids and bases form conjugate pairs
When Brønsted-Lowry acids and bases react together, they form conjugate acid-base pairs on opposite sides of the reaction equation:

A conjugate acid-base pair consists of two species that are interconverted by the transfer of a proton (H+).
- In the forward reaction, HA acts as an acid, donating a proton to form its conjugate base (A-).
- In the reverse reaction, A- acts as a base, accepting a proton from BH+ to reform the acid (HA).
- Similarly, B and BH+ form another conjugate pair, with B being the base (proton acceptor) and BH+ its conjugate acid.
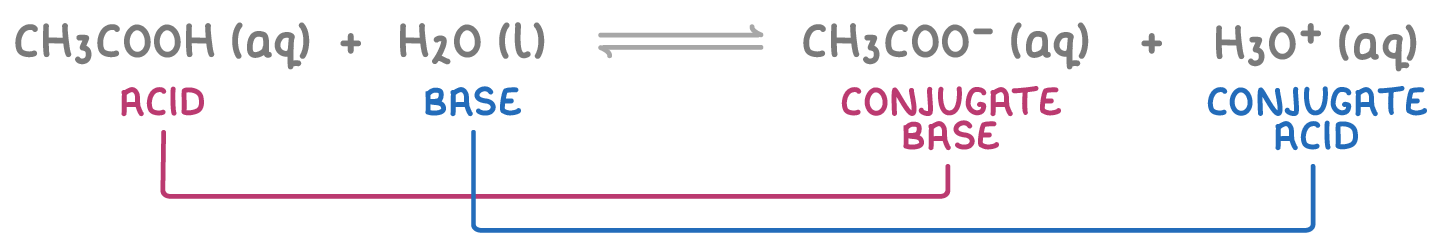
For example, when ethanoic acid (CH3COOH) reacts with water:
In this reaction:
- CH3COOH and CH3COO- are a conjugate pair. CH3COOH is the acid (proton donor) and CH3COO- is its conjugate base.
- H2O and H3O+ form the other conjugate pair, with H2O acting as the base (proton acceptor) and H3O+ as its conjugate acid.
Remember:
- The conjugate base always has one less H+ than its conjugate acid.
- The conjugate acid always has one more H+ than its conjugate base.
Water can act as an acid or a base
Water is an amphiprotic substance, meaning it can behave as both an acid and a base depending on the reaction.
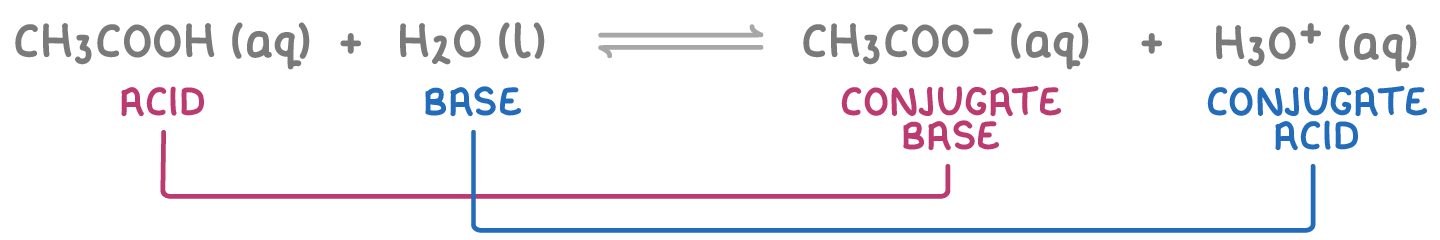
- Water as a base - When reacting with acids, water accepts a proton to form a hydronium ion (H3O+), which is water's conjugate acid. For example:
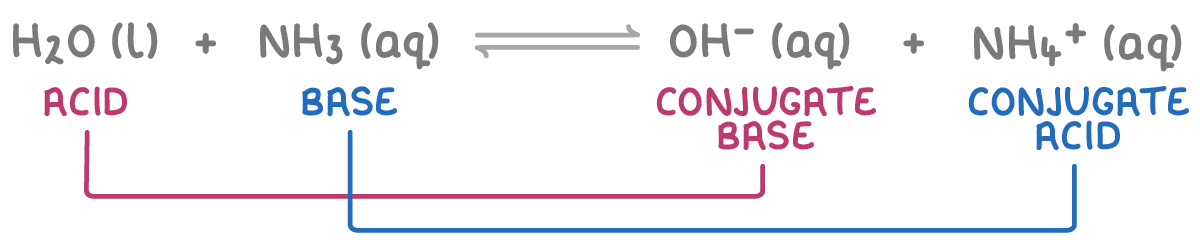
2. Water as an acid - When interacting with bases, water donates a proton to form a hydroxide ion (OH-), which is water's conjugate base. For example:
Acids react with metals and bases
1. Acids react with reactive metals to produce a salt and hydrogen gas:
- Metal + acid ➔ salt + hydrogen
- Example: Mg(s) + 2HCl(aq) ➔ MgCl2(aq) + H2(g)
2. Acids react with metal carbonates to produce a salt, water, and carbon dioxide gas:
- Metal carbonate + acid ➔ salt + water + carbon dioxide
- Example: CaCO3(s) + 2HCl(aq) ➔ CaCl2(aq) + H2O(l) + CO2(g)
3. Acids react with alkalis (soluble bases) to yield a salt and water:
- Acid + alkali ➔ salt + water
- Example: HCl(aq) + NaOH(aq) ➔ NaCl(aq) + H2O(l)
4. Acids react with insoluble bases (usually metal oxides) to form a salt and water:
- Acid + metal oxide ➔ salt + water
- Example: 2HCl(aq) + CuO(s) ➔ CuCl2(aq) + H2O(l)
Development of acid-base theories
The understanding of acid-base interactions has developed significantly over time as scientists have discovered limitations in existing models and proposed new theories:
- Lavoisier (18th century) - He proposed that all acids contain oxygen. This theory applies to acids like H2SO4, but not to HCl or H2S, which do not contain oxygen.
- Arrhenius (late 19th century) - He suggested that acids release H+ in water, whereas bases release OH-. According to this model, acids and bases react to form salt and water. However, it does not account for bases like NH3 that do not release OH-.
- Brønsted-Lowry (early 20th century) - They defined acids as proton donors and bases as proton acceptors, thereby introducing the concept of conjugate pairs. This theory built upon Arrhenius's model but expanded the definition of a base. It remains widely accepted and used today.
The ionic product of water (Kw)
A small fraction of water molecules spontaneously transfer protons between themselves, a process called autoionisation:
2H2O(l) ⇌ H3O+(aq) + OH-(aq)
Or, more simply:
H2O(l) ⇌ H+(aq) + OH-(aq)
The equilibrium constant (Kc) for this reaction is:
Kc=[H2O][H+][OH−]
However, because water is present in vast excess compared to H+ and OH-, its concentration [H2O] is essentially constant.
Multiplying both sides of the Kc expression by [H2O] gives the ionic product of water (Kw):
Kw = [H+][OH-]
At 298 K, Kw=1.00×10−14 mol2 dm−6
The value of Kw varies with temperature.
H+ and OH- concentrations in pure water
In pure water, the concentrations of H+ and OH- are equal:
Kw=[H+]2=[OH−]2=1.00×10−14 mol2 dm−6
Therefore, in pure water at 298 K:
[H+]=[OH−]=√(1.00×10−14)=1.00×10−7 mol dm−3