Organic Chemistry
This lesson covers:
- Key features that define a homologous series
- How to use general formulas to determine molecular formulas
- Common examples of important homologous series
- The structure of the carbon skeleton in organic molecules
Key features of a homologous series
Homologous compounds belong to a series of organic molecules that have the same general formula and similar chemical properties.
A homologous series has three distinctive characteristics:
- All members contain the same functional group. This specific group of atoms within a molecule is is repsonsible for the characteristic chemical reactions of that molecule.
- All memebers have the same general formula. This formula is a blueprint that can predict the molecular formula of any member in the series.
- Each subsequent compound in the series differs by a CH2 unit. This incremental addition of a CH2 group is what forms the next homologue in the series.
Determining molecular formulas
To determine the molecular formula of any member of a homologous series, you can apply its general formula. Here are two examples:
Worked example 1 - Calculating the molecular formula of an alkene
Calculate the molecular formula of an alkene with 7 carbon atoms.
Step 1: Identify the general formula for alkenes
The general formula for alkenes is CnH2n.
Step 2: Substitute the value for n (number of carbon atoms)
For an alkene with 7 carbon atoms, n = 7.
Step 3: Solve the formula to get the molecular formula
The molecular formula of the alkene = C7H2×7 = C7H14
Worked example 2 - Calculating the molecular formula of an alcohol
Calculate the molecular formula of an alcohol with 4 carbon atoms.
Step 1: Identify the general formula for alcohols
The general formula for alcohols is CnH2n+1OH.
Step 2: Substitute the value for n (number of carbon atoms)
For an alcohol with 4 carbon atoms, n = 4.
Step 3: Solve the formula to get the molecular formula
The molecular formula of the alcohol = C4H2×4+1OH = C4H9OH
Common homologous series
Here's a table outlining various homologous series, with examples for each:
| Series | Suffix/Prefix | Example |
|---|---|---|
| Alkanes | -ane | Propane (CH3CH2CH3) |
| Branched alkanes | Alkyl- | Methylpropane (CH3C(CH3)CH3) |
| Alkenes | -ene | Propene (CH3CH=CH2) |
| Haloalkanes | Fluoro- / Chloro- / Bromo- / Iodo- | Chloroethane (CH3CH2Cl) |
| Alcohols | -ol | Ethanol (CH3CH2OH) |
| Aldehydes | -al | Ethanal (CH3CHO) |
| Ketones | -one | Propanone (CH3COCH3) |
| Carboxylic acids | -oic acid | Ethanoic acid (CH3COOH) |
| Esters | Alkyl- oate | Ethyl ethanoate (CH3COOCH2CH3) |
| Amides | -amide | Ethanamide (CH3CONH2) |
| Acyl chlorides | -oyl chloride | Ethanoyl chloride (CH3COCl) |
| Arenes | -benzene / Phenyl- | Methylbenzene (C6H5CH3) |
Carbon skeletons in organic compounds
Organic compounds are built around a carbon skeleton, which is the backbone of the molecule. There are two primary types:
- Aromatic skeletons contain benzene rings, which are hexagaonal structures of carbon atoms with a ring of delocalised π-electrons.
2. Aliphatic skeletons can be either:
- Straight or branched carbon chains
- Non-aromatic carbon rings, known as alicyclic compounds
Compounds are also classified based on the types of bonds between carbon atoms:
- Saturated compounds have only single bonds between carbon atoms, like in alkanes.
- Unsaturated compounds contain double or triple bonds (C=C, C≡C), or are aromatic.
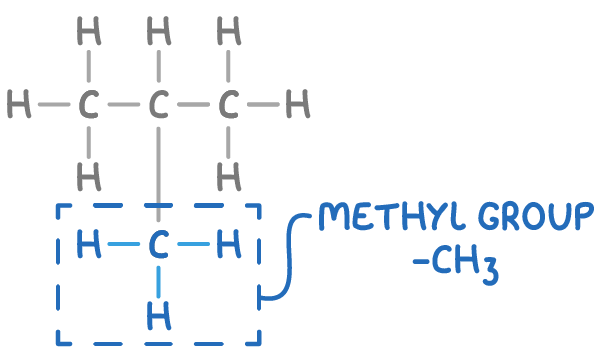
Finally, an alkyl group is a fragment of a molecule, a carbon chain with the general formula CnH2n+1.
For example, the methyl group (-CH3) is the smallest alkyl group, circled in the molecule below.