Combined Techniques
This lesson covers:
- The sequence of techniques used to determine organic structures
- Examples showing the step-by-step analysis of unknown compounds
Sequence of techniques for structure determination
To determine the structure of an unknown organic compound, chemists employ a series of analytical techniques in a specific order:
- Elemental analysis - This method determines the percentage composition of elements within a compound, enabling the calculation of the empirical formula. This formula shows the simplest ratio of elements in a compound.
- Mass spectra - The peak representing the molecular ion in a mass spectrum provides the molecular mass. This information, when combined with the empirical formula, helps deduce the molecular formula of the compound. Additionally, patterns of fragmentation can offer clues about the structure of certain molecular fragments.
- Infrared spectra - The peaks in an infrared spectrum correspond to various bond vibrations, which indicate the presence of specific bonds and functional groups within the molecule.
- NMR spectra - Nuclear magnetic resonance (NMR) spectroscopy reveals the chemical environment of hydrogen (H) and carbon (C) atoms in the compound. The splitting patterns in the spectrum can also provide information about the number of neighbouring hydrogen atoms.
Each technique builds upon the information provided by the previous ones, cumulatively offering a detailed view of the compound's structure.
Step-by-step analysis of unknown compounds
The following are two illustrative examples that demonstrate how the combined use of analytical techniques can lead to the determination of the structures of unknown compounds:
Worked example 1 - Determining the structure of an organic molecule
An unknown organic compound is analysed to determine its structure.
The following data are provided from its analysis:
- Elemental analysis: C: 60.00%, H: 13.33%, O: 26.67%
- Mass spectrum:
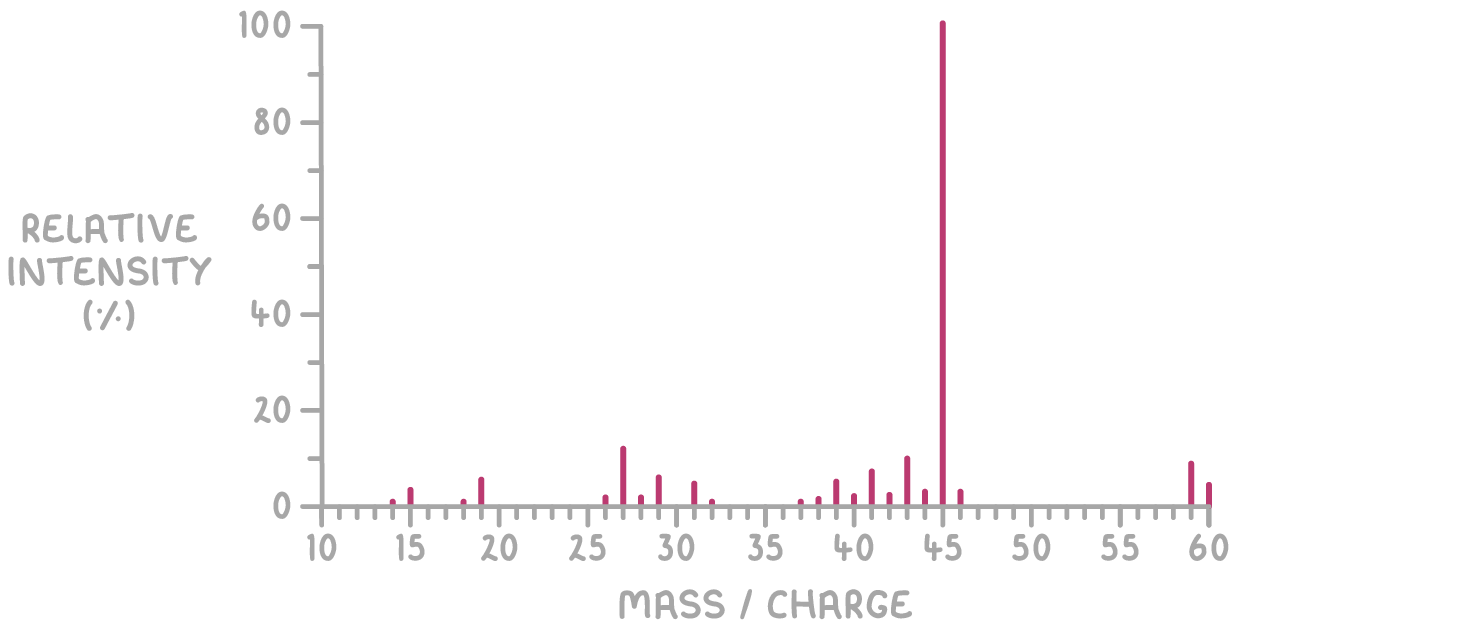
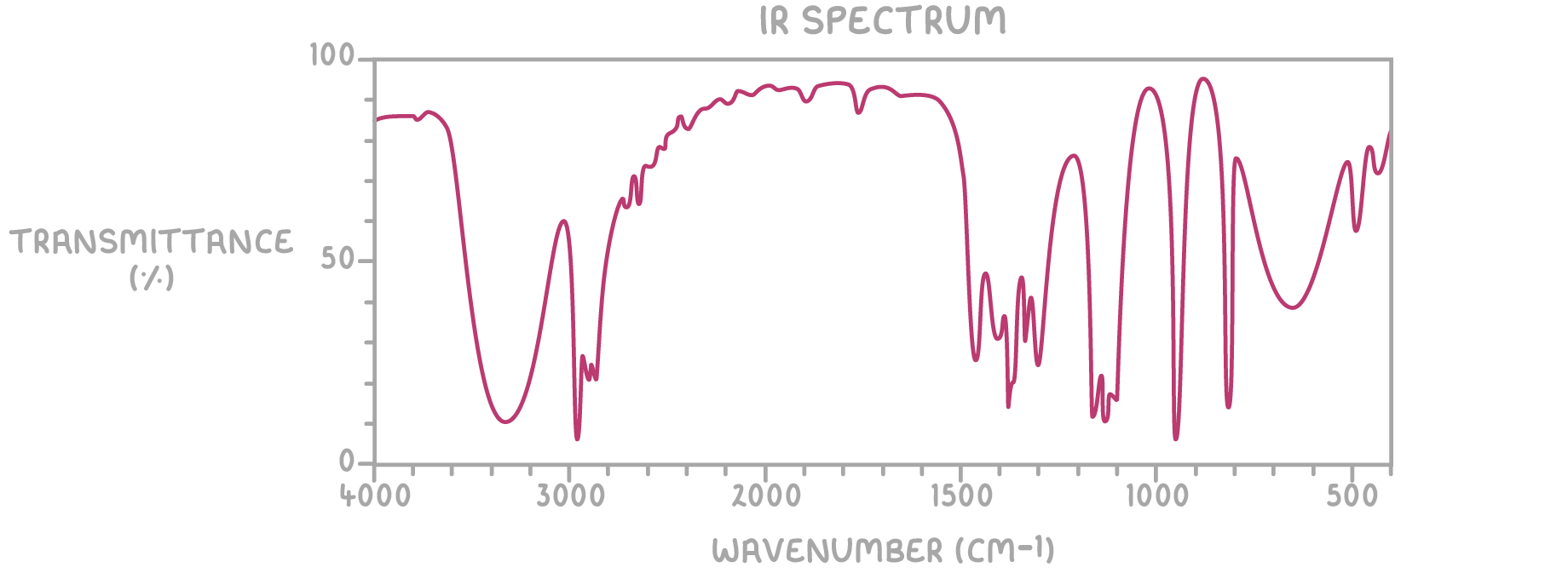
- IR spectrum:
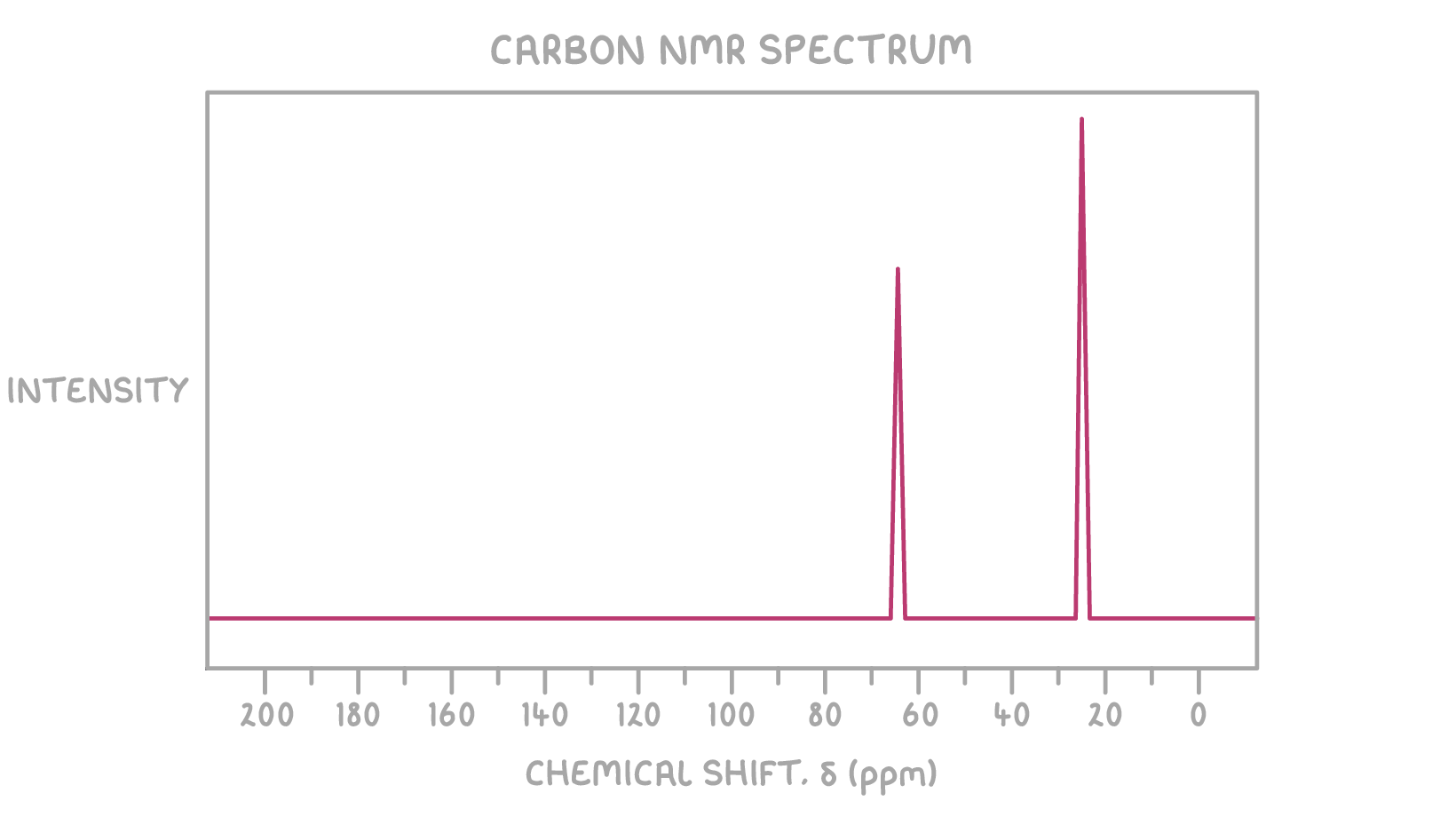
- 13C NMR spectrum:
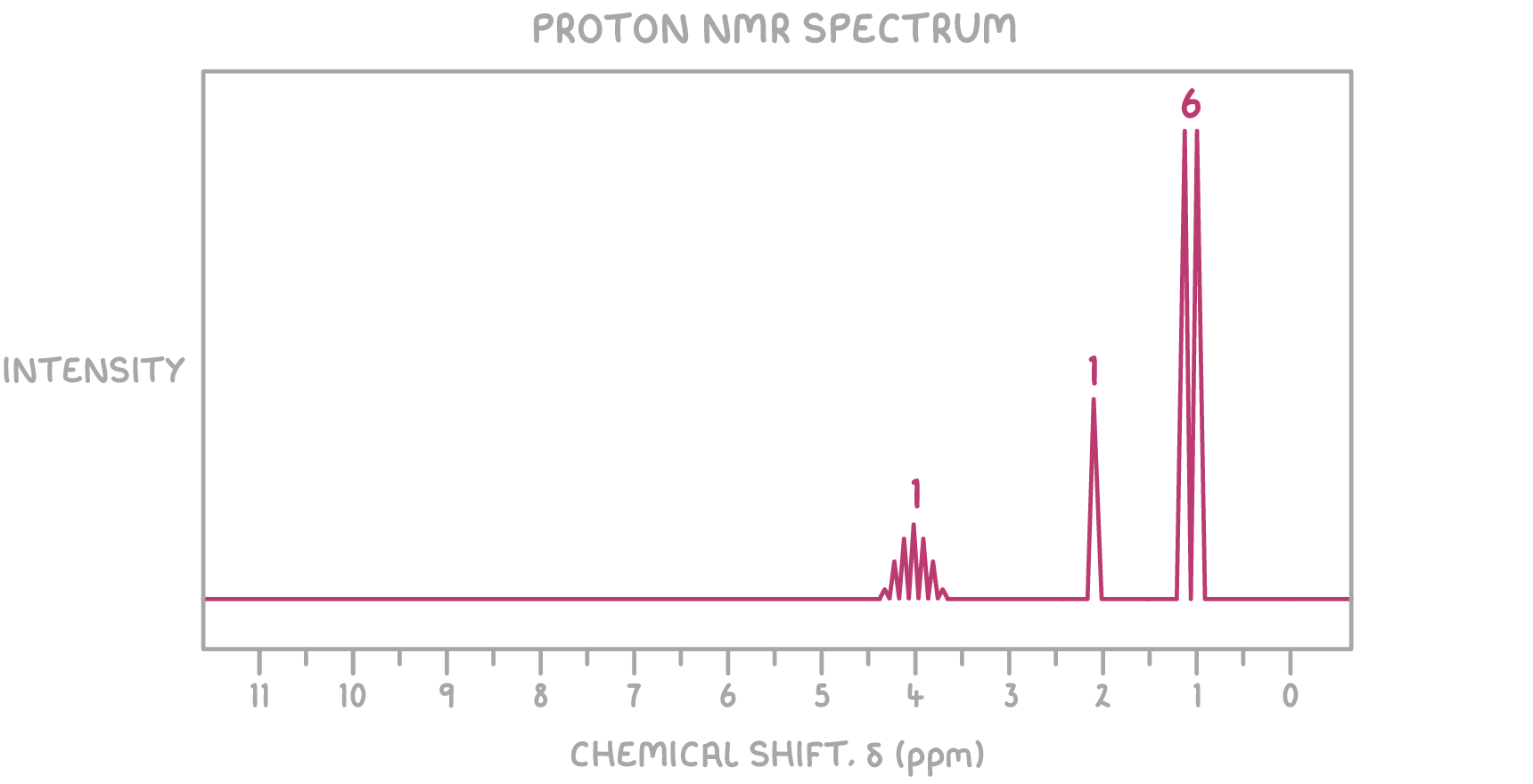
- 1H NMR spectrum:
Step 1: Elemental analysis and empirical formula calculation
Assuming 100 g of the compound for simplicity, convert the mass percentages to moles:
- Carbon: 12.060.00=5.00 mol
- Hydrogen: 1.013.33=13.33 mol
- Oxygen: 16.026.67=1.67 mol
The smallest number of moles is 1.67 (O). Dividing all by 1.67 gives:
- C: 3, H: 8, O: 1
Empirical formula is C3H8O
Step 2: Molecular formula calculation
The molecular ion peak at m/z = 60 corresponds to the molecular weight of the compound, confirming the empirical formula C3H8O as the actual molecular formula.
Step 3: IR spectrum analysis
The broad absorption at 3340 cm-1 indicates the presence of an -OH group, characteristic of alcohols.
Step 4: NMR spectrum analysis
13C NMR:
- The peak at δ 64 ppm suggests a carbon bonded to oxygen (C-O)
- The peak at δ 25 ppm suggests a carbon bonded to carbon (C-C)
1H NMR:
- The septet at δ 4.00 ppm with a relative peak area of 1 is characteristic of a single proton in a unique environment, likely attached to a carbon that is bonded to an OH group
- The doublet at δ 1.20 ppm with a relative peak area of 6 is characteristic of six protons, suggesting the presence of two methyl groups
Step 5: Structure determination
Based on all the evidence, the structure can be determined as propan-2-ol.
Worked example: 2 - Determining the structure of an organic molecule
An unknown organic compound is analysed to determine its structure.
The following data are provided from its analysis:
- Elemental analysis: C: 54.55%, H: 9.09%, O: 36.36%
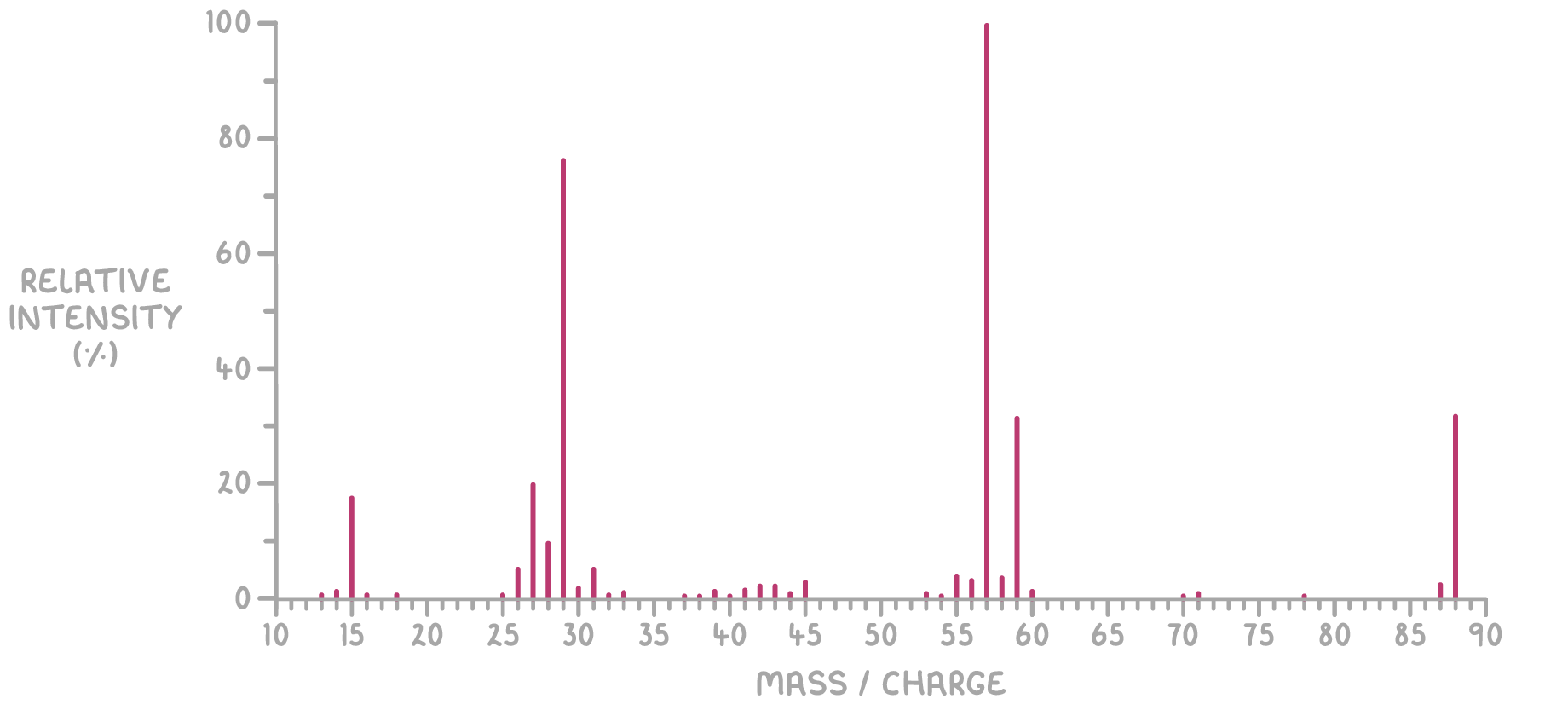
- Mass spectrum:
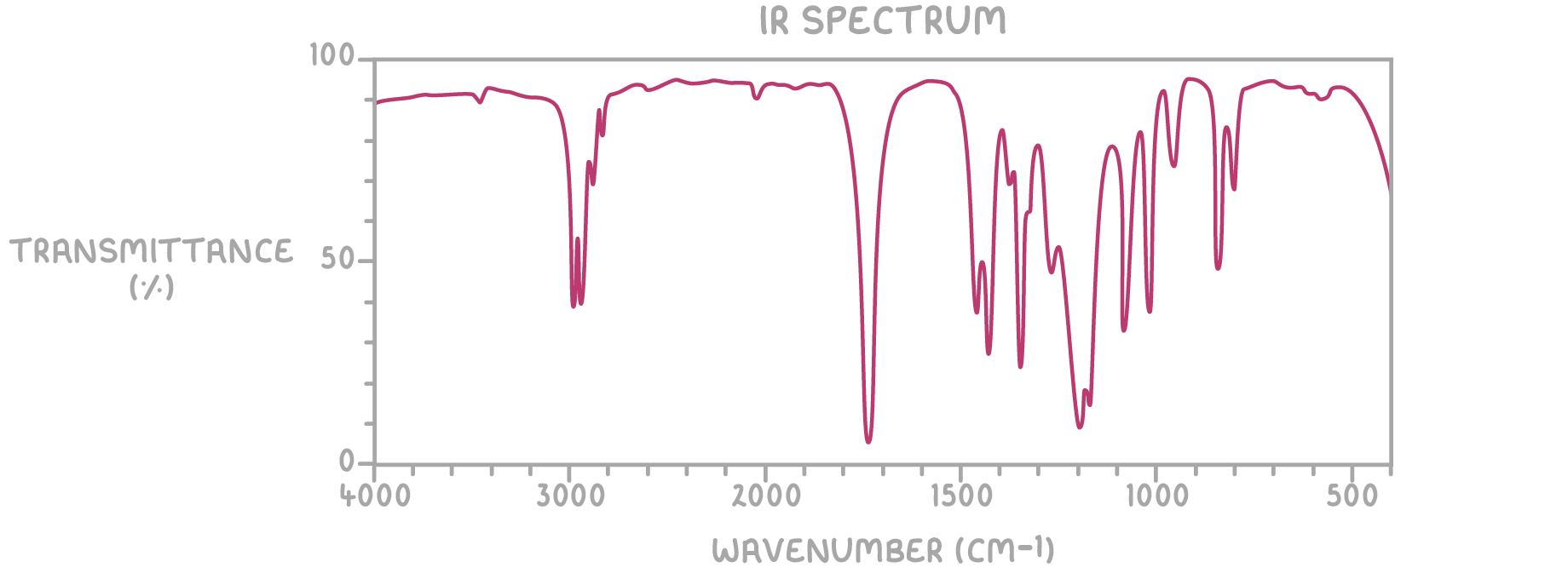
- IR spectrum:
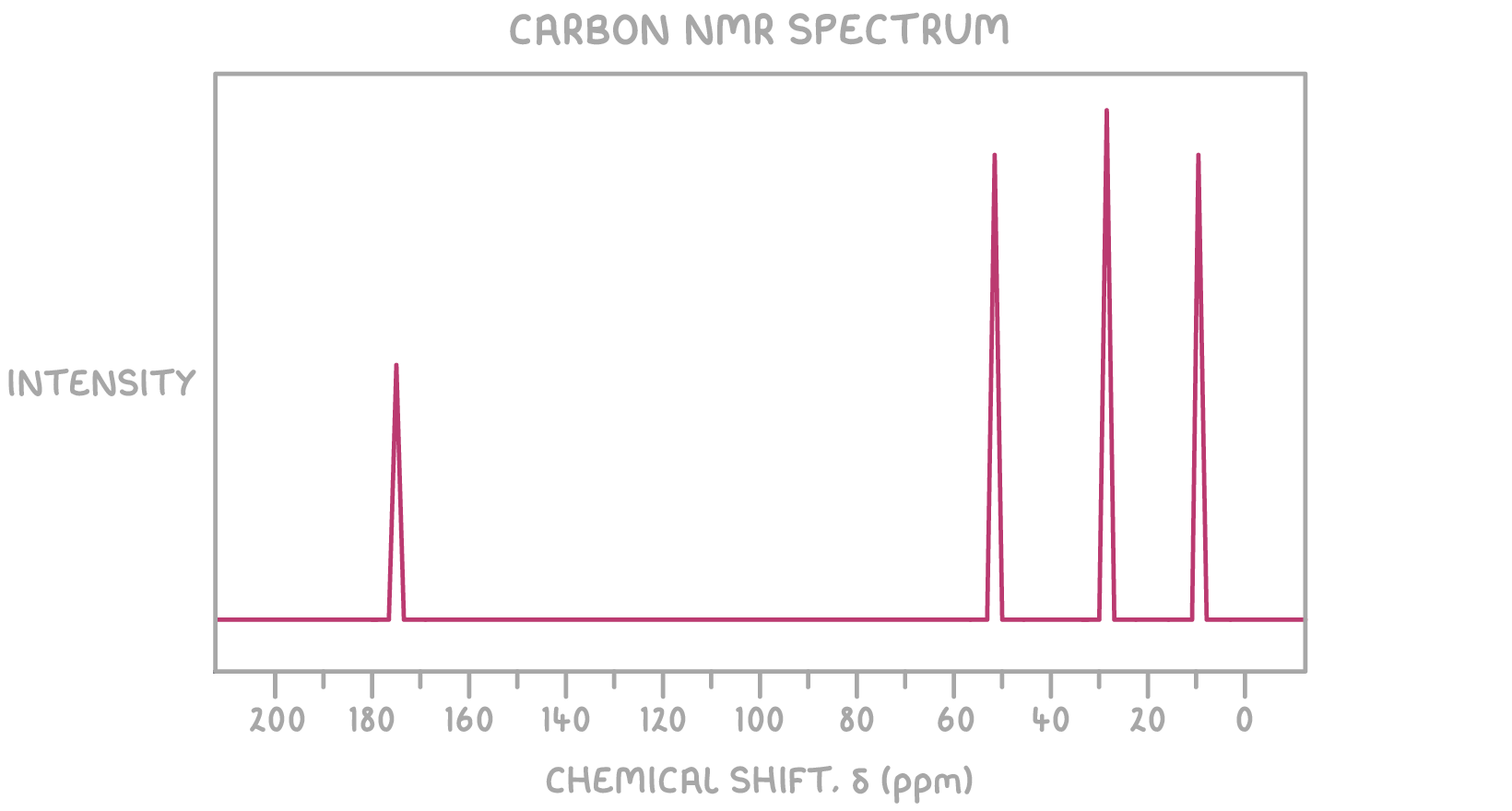
- 13C NMR spectrum:
- 1H NMR spectrum:
Step 1: Elemental analysis and empirical formula calculation
Assuming 100 g of the compound for simplicity, convert the mass percentages to moles:
- Carbon: 12.054.55=4.55 mol
- Hydrogen: 1.09.09=9.09 mol
- Oxygen: 16.036.36=2.27 mol
The smallest number of moles is 2.27 (O). Dividing all by 2.27 gives:
- C: 2, H: 4, O: 1
Empirical formula is C2H4O
Step 2: Molecular formula calculation
The molecular weight from the molecular ion peak (m/z = 88) matches twice the weight of the empirical formula (2×442×44). Thus, the molecular formula is C4H8O2
Step 3: IR spectrum analysis
The strong absorption at 1,740 cm-1 suggests a carbonyl (C=O) group.
Step 4: NMR spectrum analysis
13C NMR:
- The peak at δ 175 ppm suggests a carbonyl (C=O) carbon
- The peak at δ 52 ppm suggests a carbon bonded to oxygen (C-O)
- The peak at δ 9 ppm suggests a carbon bonded to carbon (C-C)
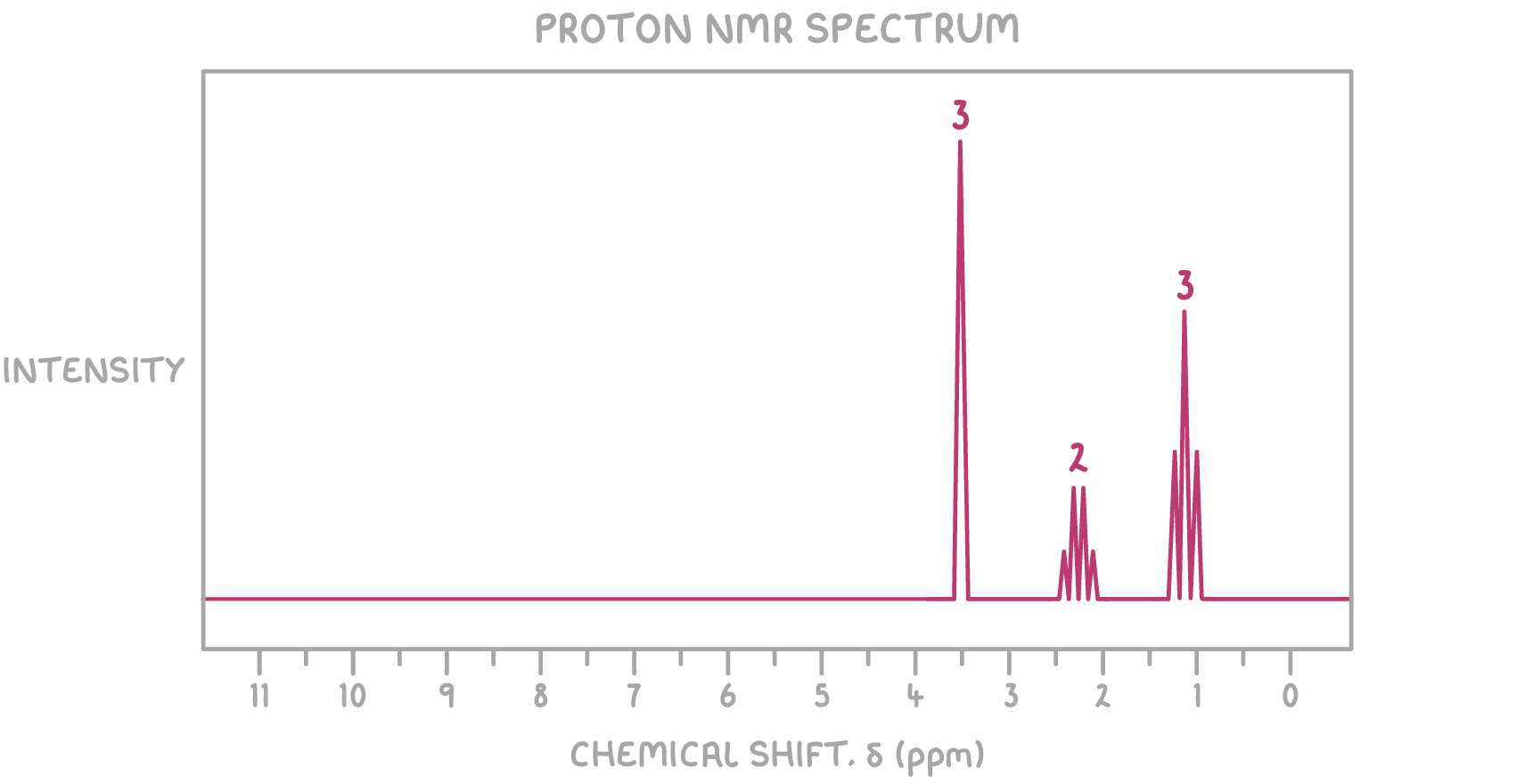
1H NMR:
- The singlet at δ 3.70 ppm with a relative peak area of 3 is characteristic of a methyl group (CH3) adjacent to an oxygen atom
- The quartet at δ 2.32 ppm with a relative peak area of 2 is characteristic of a CH2 group adjacent to a carbonyl group on one side and a methyl group on the other side
- The triplet at δ 1.15 ppm with a relative peak area of 3 is characteristic of a methyl group (CH3) adjacent to a CH2 group
Step 5: Structure determination
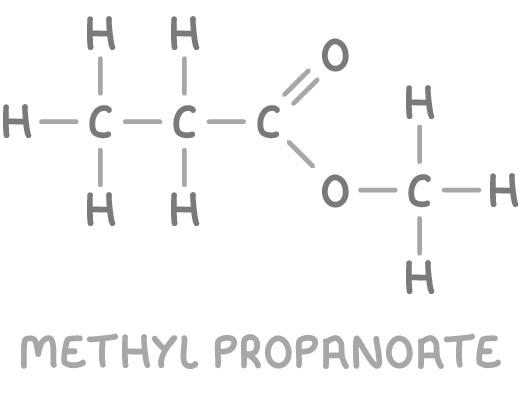
Based on all the evidence, the structure can be determined as methyl propanoate.