Acyl Chlorides
This lesson covers:
- The structure and nomenclature of acyl chlorides
- How acyl chlorides are made from carboxylic acids
- Reactions of acyl chlorides
Acyl chlorides contain the functional group -COCl
Acyl chlorides, also known as acid chlorides, are a type of compound that features the functional group -COCl. These compounds are derived from carboxylic acids, where the hydroxyl (-OH) group is replaced by a chlorine atom (-Cl).
Similar to aacyl chlorides, acid anhydrides and amides are also derivatives of carboxylic acids.

To name an acyl chloride, the suffix "-oic acid" of the parent carboxylic acid is replaced with "-oyl chloride". For example:
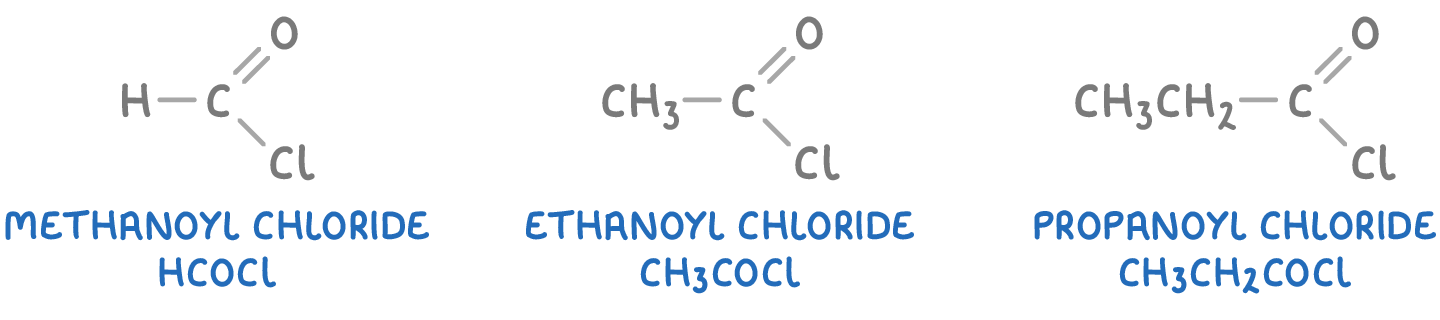
When numbering the carbon chain in an acyl chloride, the numbering starts from the end closest to the -COCl group, similar to carboxylic acids.
Preparing acyl chlorides using SOCl2
One common method to prepare acyl chlorides is by reacting carboxylic acids with thionyl chloride (SOCl2) at room temperature according to the equation:
RCOOH + SOCl2 ➔ RCOCl + SO2 + HCl
For instance, ethanoic acid reacts with SOCl2 to yield ethanoyl chloride:
CH3COOH + SOCl2 ➔ CH3COCl + SO2 + HCl
Thionyl chloride is especially useful for this reaction because the by-products, SO2 and HCl, are gaseous and escape the reaction mixture, driving the reaction towards completion.
Reactions of acyl chlorides
Acyl chlorides are highly reactive due to their polar C=O bond and the chlorine atom, which is readily displaced.
They can react with:
- Water - This reaction is vigorous, even at low temperatures, and reforms the carboxylic acid. The reaction also produces steamy fumes of hydrogen chloride gas. For example, ethanoyl chloride is hydrolysed to ethanoic acid according to the equation:
CH3COCl + H2O ➔ CH3COOH + HCl
- Alcohols - At room temperature, this reaction is vigorous, producing an ester and HCl. For example, ethanoyl chloride reacts with ethanol to form ethyl ethanoate according to the equation:
CH3COCl + C2H5OH ➔ CH3COOC2H5 + HCl
- Ammonia - This reaction occurs violently at room temperature, resulting in a primary amide and NH4Cl. For example, ethanoyl chloride reacts with ammonia to form ethanamide according to the equation:
CH3COCl + 2NH3 ➔ CH3CONH2 + NH4Cl
- Primary amines - This reaction occurs violently at room temperature, resulting in a secondary amide and (CH3)2NH2+Cl-. For example, ethanoyl chloride reacts with methylamine to form N-methylethanamide according to the equation:
CH3COCl + 2CH3NH2 ➔ CH3CONHC2H5 + (CH3)2NH2Cl
- Phenol - Though slower, this reaction at room temperature is useful for forming phenolic esters, esterifying the typically unreactive phenol. For example, ethanoyl chloride reacts with phenol to form phenyl ethanoate according to the equation:
CH3COCl + C6H5OH ➔ CH3COOC6H5 + HCl