Stereoisomerism
This lesson covers:
- Why double bonds restrict rotation in alkenes
- Definition of stereoisomers
- E-Z stereoisomers in alkenes
- Assigning E-Z configuration using Cahn-Ingold-Prelog rules
- When E-Z stereoisomers can also be called cis-trans
Double bonds restrict rotation in alkenes
Alkenes are characterised by the presence of carbon-carbon double bonds, which significantly influence their molecular structure:
- The atoms connected to each of the doubly bonded carbons are positioned in the same plane as these carbon atoms. This planar arrangement is the result of sideways overlapping of the p orbitals, forming the π bond.
- Around the carbon-carbon double bonds, rotation is restricted due to the stable overlapping of the p orbitals that form the π bond.
- It's important to note that while double bonds restrict rotation, single bonds in the molecule can still rotate freely.
Stereoisomers have different spatial arrangements of atoms.
Stereoisomers have the same molecular formula and the same connectivity of atoms but a different three-dimensional arrangement of atoms.
These isomers typically occur in alkenes where each of the carbon atoms in the double bond has two different groups attached.
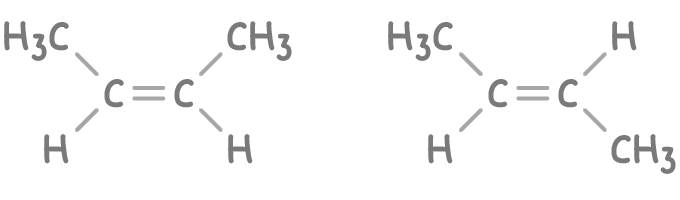
For example, the stereoisomers of but-2-ene are shown below.
The E-Z system classifies stereoisomers in alkenes
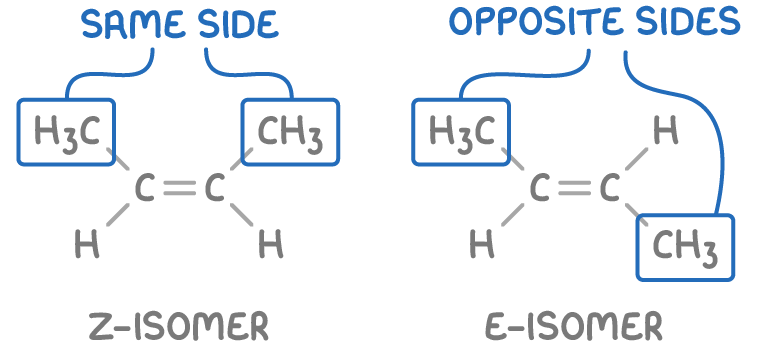
Alkene stereoisomers can be classified into two types:
- Z-isomer - Groups of interest are on the same side of the double bond.
- E-isomer - Groups of interest are positioned across from each other on opposite sides of the double bond.
Explanation:
- "Z" stands for Zusammen, a German word meaning “together”.
- "E" stands for Entgegen, a German word meaning “opposite”.
In the case of but-2-ene stereoisomers:
Assigning E-Z configuration using Cahn-Ingold-Prelog rules
The Cahn-Ingold-Prelog (CIP) rules help in assigning the E-Z configuration to alkenes.
Step 1 - Priority ranking
Assign priority to the groups attached to each of the double-bonded carbons based on the atomic number. The group with the highest atomic number receives the highest priority.
Step 2 - Assign E-Z configuration
With priorities set, label the stereoisomer with the highest-ranking groups across the C=C bond as the E-isomer. The stereoisomer with the highest-ranking groups on the same side of the bond is the Z-isomer.
Special cases:
- If the attached atoms are of the same atomic number, the priority is determined by considering the next atom in the chain.
- In alkenes with only three distinct groups, priority is based on the positions of the highest-priority atoms alone.
Worked example 1 - Assigning E-Z configurations
Assign an E-Z configuration to the stereoisomer of 1-bromo-2-chloroethene shown below.
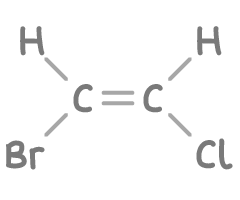
Step 1: Priority ranking
- On the left side of the double bond, compare the Br atom with the H atom. Bromine (Br) has a higher atomic number than hydrogen (H), so Br gets higher priority.
- On the right side of the double bond, compare the Cl atom with the H atom. Chlorine (Cl) has a higher atomic number than hydrogen (H), so Cl gets higher priority.
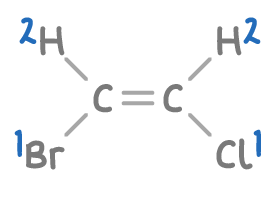
Step 2: Assign E-Z configuration
- With Br and Cl as the highest priority groups, we check their positions.
- Since the high-priority groups (Br and Cl) are on the same side of the double bond, this molecule is assigned the Z-configuration.
Worked example 2 - Assigning E-Z configurations
Assign an E-Z configuration to the stereoisomer of 1-bromo-1-fluoro-2-methylbut-1-ene shown below.
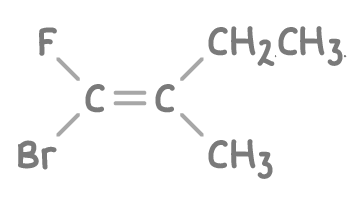
Step 1: Priority ranking
- On the left side of the double bond, compare the Br atom with the F atom. Bromine (Br) has a higher atomic number than fluorine (F), so Br gets higher priority.
- On the right side of the double bond, compare the methyl (CH3) group with the ethyl (CH2CH3) group. The methyl carbon is attached only to hydrogen atoms whereas the ethyl carbon is bonded to another carbon. Carbon (C) has a higher atomic number than hydrogen (H), so CH2CH3 gets higher priority.
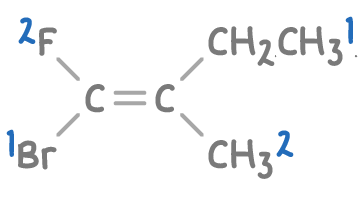
Step 2: Assign E-Z configuration
- With Br and CH2CH3 as the highest priority groups, we check their positions.
- Since the high-priority groups (Br and CH2CH3) are on opposite sides of the double bond, this molecule is assigned the E-configuration.
The cis-trans system applies when groups are identical
The cis-trans naming system is an alternative to the E-Z system for E-Z stereoisomers, applicable when the double-bonded carbons share at least one identical group.
- Cis - Identical groups are on the same side.
- Trans - Identical groups are on opposite sides.
Thus, Z-but-2-ene equates to cis-but-2-ene, and E-but-2-ene to trans-but-2-ene.
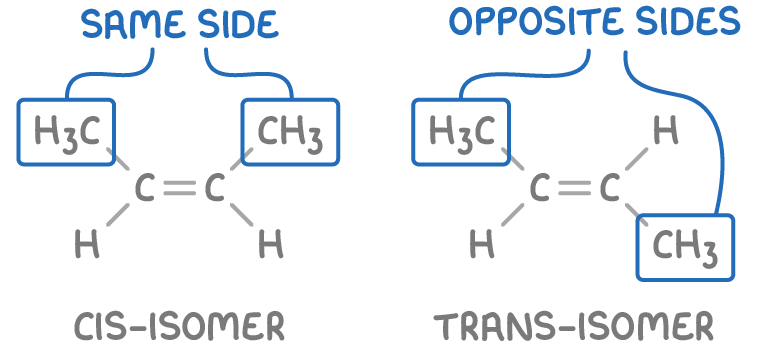
Note: The cis/trans system is not applicable when the groups attached to the double-bonded carbons are completely different.