Polymerisation in Alkenes
This lesson covers:
- How alkenes can polymerise to form addition polymers
- Drawing and naming addition polymers
- The issues of plastic waste and options for disposal
- Developments in biodegradable and photodegradable polymers
Alkenes polymerise by opening their double bonds
Alkenes contain a carbon-carbon double bond.
This bond can open up, allowing alkene molecules to join end-to-end and form long chains called polymers.
- The individual alkene molecules are called monomers.
- Joining many monomers together is called polymerisation.
- Polymers made this way are called addition polymers because the double bond opens up and monomer units are added to the chain.
For example, ethene monomers polymerise to form the addition polymer poly(ethene):
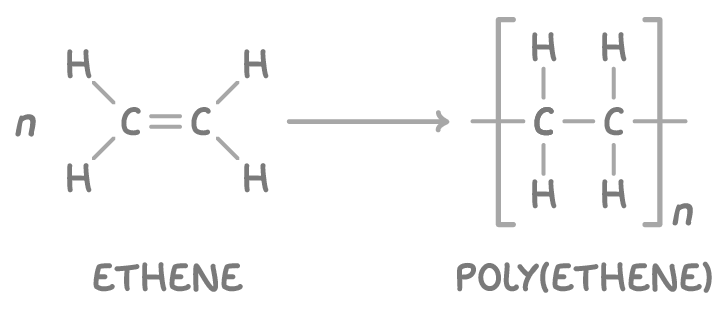
Drawing polymer repeating units
Polymers are made up of smaller repeating units. A repeating unit is the smallest section of a polymer chain that repeats over and over.
To draw the repeating unit from given monomer:
- Replace the carbon-carbon double bond with a single bond.
- Extend single bonds from each carbon atom to represent attachment sites within the polymer chain.
For example, the repeating unit of poly(propene) can be deduced from the propene monomer.
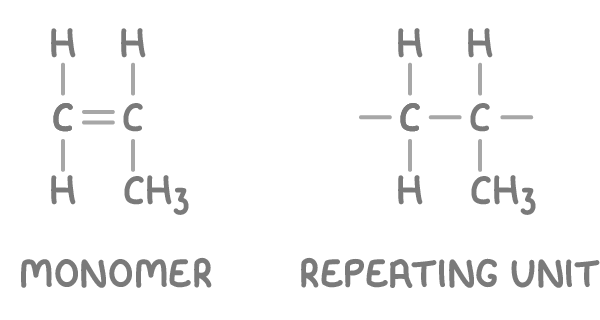
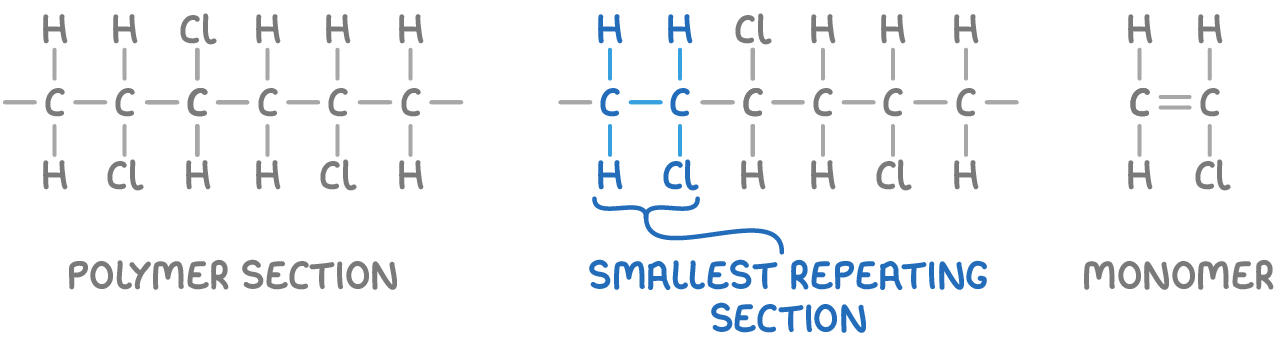
To deduce the monomer from a given polymer section:
- Identify the smallest section that repeats in the full polymer chain.
- Replace the carbon-carbon single bond with a double bond.
For example, the monomer of poly(chloroethene) can be deduced from a section of the polymer chain.
Naming addition polymers
Addition polymers made from alkenes follow systematic naming rules:
- Take the name of the alkene monomer.
- Enclose the monomer name in brackets.
- Add the prefix "poly".
For example, the addition polymer made from butene is poly(butene).
Addition polymers made from alkenes are called polyalkenes.
Dealing with the accumulation of waste plastics
Plastic waste is a major environmental issue with over two million tonnes produced yearly in the UK alone. The non-biodegradable nature of most plastics, due to their chemically inert structure, exacerbates this issue.
There are several approaches to dealing with plastic waste:
- Incineration
- Recycling
Incineration
Incinerating waste plastics in specialised facilities can generate useful heat or electricity.

| Advantages | Disadvantages |
|---|---|
| 1. Recovers energy from waste plastics, reducing reliance on fossil fuels | 1. Generates toxic waste products, such as HCl, which require costly removal processes to prevent environmental damage |
| 2. Substantially reduces the volume of plastic waste, minimising landfill requirements | 2. Releases greenhouse gases, primarily CO2, contributing to climate change |
| 3. Incineration is an energy intensive process, partly offsetting the energy recovered |
Recycling
Recycling waste plastics can be achieved through two main approaches:
- Mechanical recycling - This involves sorting, cleaning, and remoulding waste plastics into new objects without significantly altering the chemical structure of the polymer.
- Feedstock recycling - This process breaks down polymers into their constituent monomers through cracking. These monomers can then be used as raw materials for the synthesis of new plastics and other organic chemicals.

| Advantages | Disadvantages |
|---|---|
| 1. Conserves valuable crude oil resources by reducing the need for raw materials | 1. Requires complex and costly multi-step processes for sorting, cleaning, and reprocessing waste plastics |
| 2. Typically results in lower CO2 emissions compared to incineration and production from raw materials | 2. Contamination from additives, dyes, or other polymers can compromise the quality and performance of recycled products |
| 3. Can be more economical than producing new polymers, especially when oil prices are high | 3. Recycled plastics may have limited applications due to reduced mechanical properties and potential contamination issues |
| 4. Reduces the amount of waste sent to landfills |
Developments in degradable polymers
To address the challenges posed by non-biodegradable polymers, researchers have developed degradable polymers that decompose more quickly, offering environmental benefits by reducing the accumulation of persistent plastic waste.
Biodegradable polymers:
- Incorporate starch granules or are derived from plant-based monomers, making them more susceptible to digestion by microorganisms.
- Polyamides and polyesters can decompose through acid hydrolysis under the hot, acidic conditions often found in landfills.
Photodegradable polymers:
- Contain light-sensitive carbonyl groups that absorb UV light, weakening nearby bonds and causing the polymer to fragment into smaller pieces.
- Fragmentation accelerates the biodegradation process.
- Have limited effectiveness in landfill environments where waste is typically buried, blocking light and preventing photodegradation.