The Chemistry of Phenol
This lesson covers:
- The structure of phenols
- Why phenols are more reactive than benzene
- Reactions of phenols
Phenols contain benzene rings with -OH groups
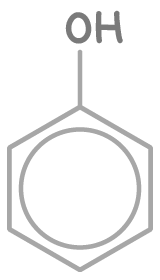
Phenol has the formula C6H5OH. The key structural feature of a phenol is an aromatic benzene ring attached to a hydroxyl (-OH) group.
Other phenolic compounds have additional substituents bonded to the benzene ring.
The skeletal formula of phenol is:
Phenol is more reactive than benzene
Phenol is more reactive towards electrophilic substitution than benzene because:
- The electron-donating -OH group activates the aromatic ring, making it more susceptible to electrophilic attack.
- One of the lone pairs of electrons in a p-orbital of the oxygen atom overlaps with the delocalised π system of electrons in the benzene ring.
- This allows the lone pair to partially delocalise into the aromatic π system, increasing the electron density within the ring.
- A higher electron density makes the ring more reactive towards electrophiles.
Reactions of phenols
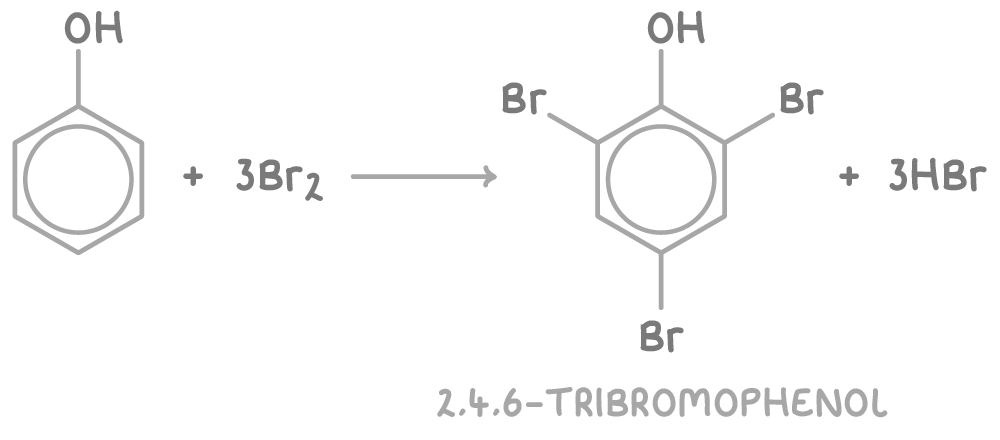
With bromine water:
- Phenol causes bromine water to decolourise as substitution occurs at the 2- and 4- positions, producing 2,4,6-tribromophenol as a white precipitate.
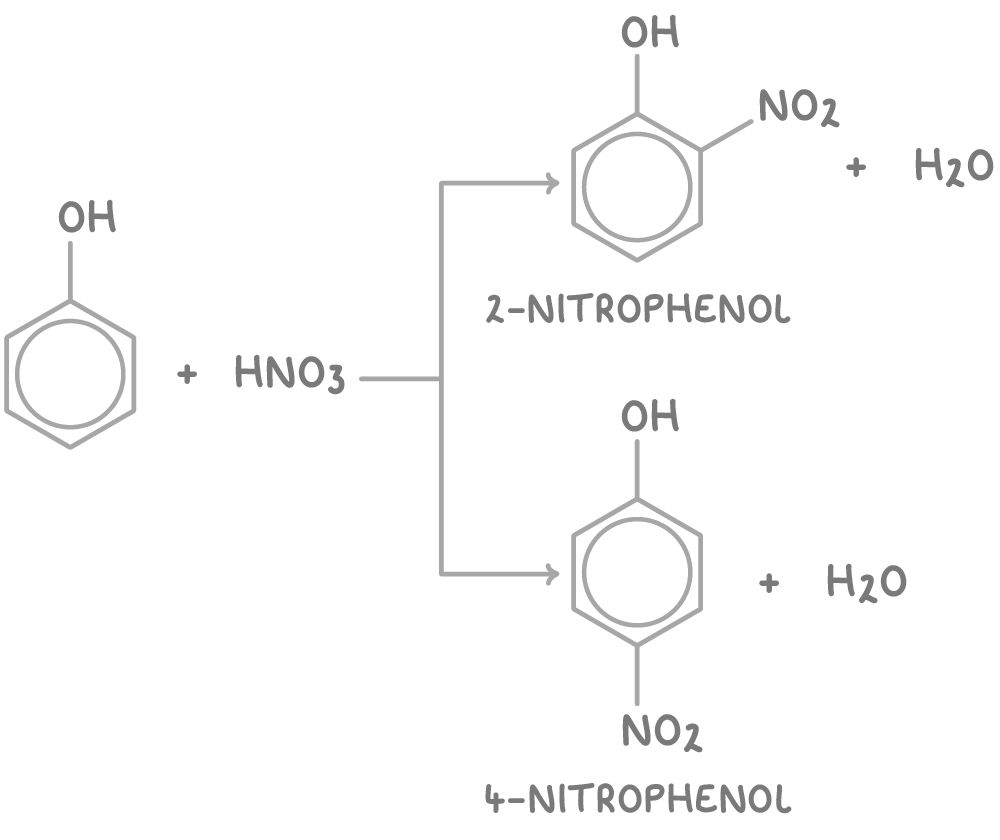
With dilute nitric acid:
- Direct nitration of phenol yields two main isomers, 2-nitrophenol and 4-nitrophenol, at the 2- and 4- positions respectively.
With bases:
- Phenol reacts with NaOH(aq) in a neutralisation reaction to form sodium phenoxide and water:
C6H5OH + NaOH ➔ C6H5ONa + H2O
- Phenol does not react with Na2CO3(aq) due to its relatively weak acidity.
To distinguish a phenol from a carboxylic acid, you can test its reaction with sodium carbonate (Na2CO3); phenols do not react with Na2CO3, while carboxylic acids do to produce effervescence due to the release of carbon dioxide gas.
Bromination and nitration of phenol requires milder conditions than benzene
The presence of the -OH group in phenol activates the aromatic ring, enhancing its electron density and allowing for electrophilic attacks under more moderate conditions than required for benzene. This characteristic explains why phenol undergoes electrophilic nitration and bromination more readily.
The reaction conditions for both benzene and phenol during bromination and nitration illustrate the differences in their reactivity:
| Reaction | Benzene | Phenol |
|---|---|---|
| Bromination | Br2, FeBr3 catalyst, room temperature | Br2(aq), room temperature |
| Nitration | Conc. HNO3, conc. H2SO4, heat | Dilute HNO3, room temperature |