Organohalogen Compounds in the Environment
This lesson covers:
- What chlorofluorocarbons (CFCs) are
- How CFCs reach the ozone layer
- The role of chlorine radicals in ozone depletion
- Additional causes of ozone depletion
Chlorofluorocarbons are stable halogenoalkanes
Chlorofluorocarbons (CFCs) are a type of halogenoalkane containing only carbon, chlorine and fluorine.
Key features include:
- They contain no C-H bonds as all hydrogen atoms are substituted by chlorine and fluorine.
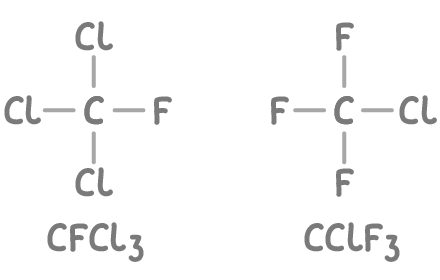
- Common examples are CCl3F (trichlorofluoromethane) and CClF3 (chlorotrifluoromethane).
CFCs were widely used industrially and domestically as refrigerants, propellants and solvents until the late 1980s.
Their widespread use was due to properties like:
- High stability
- Low toxicity
- Non-flammability
- Volatility
Their high stability is a result of the strong carbon-halogen bonds.
How CFCs reach the ozone layer
Ozone (O3) in the upper atmosphere acts as a protective barrier by absorbing harmful ultraviolet (UV) radiation from the sun. This "ozone layer" prevents damage to humans and ecosystems.
While very stable at ground level, CFC molecules can survive long enough in the atmosphere to be carried up into the stratosphere. High-energy UV radiation breaks the carbon-halogen bond, releasing chlorine radicals. For example,
CF2Cl2 ➔ CF2Cl• + Cl•
Chlorine radicals destroy ozone
Chlorine radicals initiate a chain reaction that rapidly depletes ozone through a series of propagation reactions:
- UV radiation forms chlorine radicals (Cl•) which react with ozone molecules:
Cl• + O3 ➔ ClO• + O2
- The chlorine radical is regenerated:
ClO• + O ➔ Cl• + O2
The overall effect of these reactions is:
O3 + O ➔ 2O2
This cycle allows a single chlorine radical to destroy more than 10,000 ozone molecules. The resulting depletion forms "holes" in the ozone layer, increasing UV radiation penetration.
Other ozone depleters
Chlorine radicals are not the only cause of ozone destruction. Nitrogen oxide radicals also participate in similar catalytic reactions to break down ozone.
Major sources of nitrogen oxides include lightning storms and vehicle internal combustion engines.
The propagation reactions between nitrogen monoxide radicals and ozone are:
- UV radiation forms nitrogen monoxide radicals (NO•) which react with ozone molecules:
NO• + O3 ➔ NO2• + O2
- The nitrogen monoxide radical is regenerated:
NO2• + O ➔ NO• + O2
So both chlorine and nitrogen oxide radicals deplete ozone, allowing more UV radiation to reach Earth.