Acid-base Titrations
This lesson covers:
- What titrations are and how they allow you to determine neutralisation quantities
- How to accurately carry out a titration using pipettes and burettes
- The use of indicators to identify the end point
- Creating standard solutions for use in titrations
Titrations determine neutralisation volumes
Titration is an analytical method used to determine the concentration of a solution of known volume by gradually adding a solution of known concentration to it until neutralisation occurs.
- A solution with an unknown volume and concentration is referred to as the analyte. It is measured using a pipette and placed into a conical flask.
- The standard solution, which has a known concentration, is filled into a burette and slowly added to the analyte.
- To visually determine when neutralisation has occurred, an indicator is added to the flask, causing a colour change at the end point - the point at which the reaction has just reached completion and neither reactant is in excess.
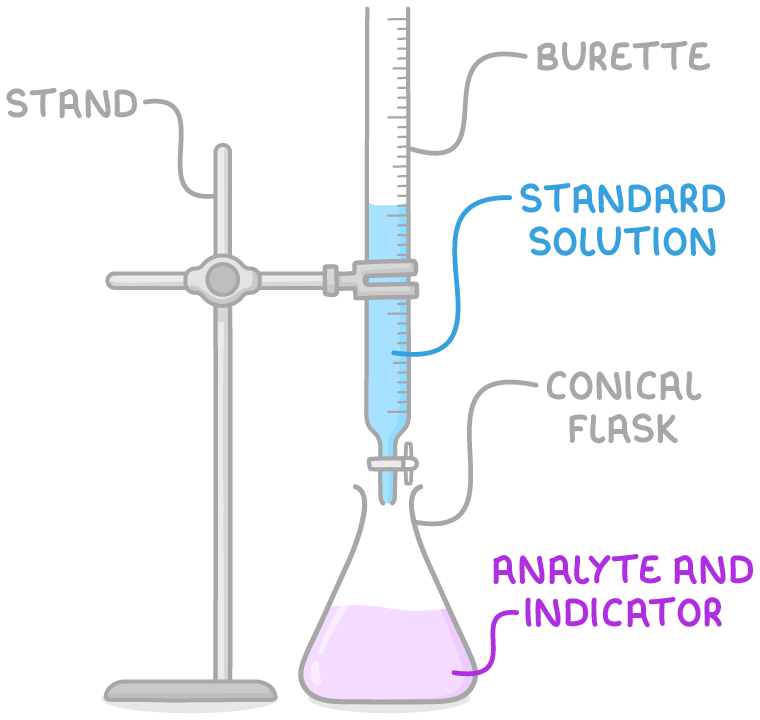
By calculating the volume of the standard solution required to reach the end point, you can determine the concentration of the analyte.
Steps in carrying out a titration
To perform a titration accurately, follow these steps:
- Measure the analyte into a conical flask using a pipette, and add an indicator.
- Fill the burette with the standard solution and note the initial reading.
- Gradually add the standard solution to the flask, swirling to mix, until you approximately reach the point of neutralisation.
- Refill the burette and repeat steps 2 and 3 to get an approximate idea of where neutralisation occurs.
- Conduct a precise titration by adding the standard solution slowly to identify the exact end point. Record the final burette reading, also known as the titre volume.
- Perform the titration multiple times to obtain concordant results, which are titre volumes that differ by no more than 0.10 cm3.
- Calculate the mean titre volume using the concordant results.
Indicators mark reaction completion
Indicators are crucial for visually identifying the end point of a titration through a colour change, indicating neutralisation.
Common indicators include:
- Methyl orange, which changes from yellow to red.
- Phenolphthalein, which changes from pink to colourless.
Conduct titrations against a white background to easily observe the colour change.
Standard solutions enable calculation of unknown concentrations
To determine an unknown concentration via titration, it's essential to use a standard solution with a precisely known concentration.
These solutions are prepared by:
- Weighing a precise mass of solute using a balance.
- Dissolving the weighed solute in distilled water in a beaker.
- Transferring the solution to a volumetric flask of a specific volume.
- Rinsing the beaker several times with distilled water and adding the rinsings to the volumetric flask.
- Diluting the solution to the mark on the volumetric flask with a pipette.
- Inverting the flask several times to ensure thorough mixing of the solution.
The formula for the concentration of the stock solution is:
concentration in g dm−3 =volume in dm3mass of solute in g
Worked example 1 - Determining the mass of solid Na2CO3 needed
What mass in g of solid sodium carbonate (Na2CO3) is needed to make 250 cm3 of a 0.400 mol dm-3 solution?
Step 1: Conversion of cm3 into dm3
To convert from cm3 into dm3, divide by 1,000
250 cm3 = 0.250 dm3
Step 2: Calculate number of moles of Na2CO3 required
n = c x V = 0.400 x 0.250 = 0.100 mol
Step 3: Calculate mass of Na2CO3 required
m = n x Mr = 0.100 x 106.0 = 10.6 g