Moles and Gas Volumes
This lesson covers:
- The molar gas volume
- Measuring the molar volume of a gas experimentally
- Calculating gas volumes using molar calculations
- Calculating gas volumes using reaction stoichiometry
The molar gas volume
The molar gas volume is:
- The volume occupied by one mole of any gas a constant temperature and pressure.
- Measured in dm3 mol-1.
- Denoted as Vm.
Under standard room temperature and pressure (r.t.p.) conditions:
- All gases possess the same molar volume of 24 dm3 mol-1.
- r.t.p. corresponds to 293 K (20°C) and 101 kPa.
To determine the number of moles in a given gas volume, use the formula:
n =VmV
Where:
- n = number of moles of gas (mol)
- V = volume of gas (dm3)
- Vm = molar volume (dm3 mol-1)
Worked example 1 - Calculating number of moles
Calculate the number of moles of carbon dioxide (CO2) in a 2.5 dm3 container at r.t.p.
Step 1: Equation
n =VmV
Step 2: Substitution and correct evaluation
n =242.5=0.10 mol
Worked example 2 - Calculating volume
Calculate the volume of nitrogen gas (N2) in dm3 at r.t.p corresponding to 0.50 moles of N2.
Step 1: Rearrange equation
V = n x Vm
Step 2: Substitution and correct evaluation
V = 0.50 x 24 = 12 dm3
Measuring molar volumes experimentally
The volume of a gas produced in a reaction can be measured either by a gas syringe or by water displacement. This data allows calculation of the molar volume.
For example, in the reaction:
CaCO3(s) + 2HCl(aq) ➔ CaCl2(aq) + CO2(g) + H2O(l)
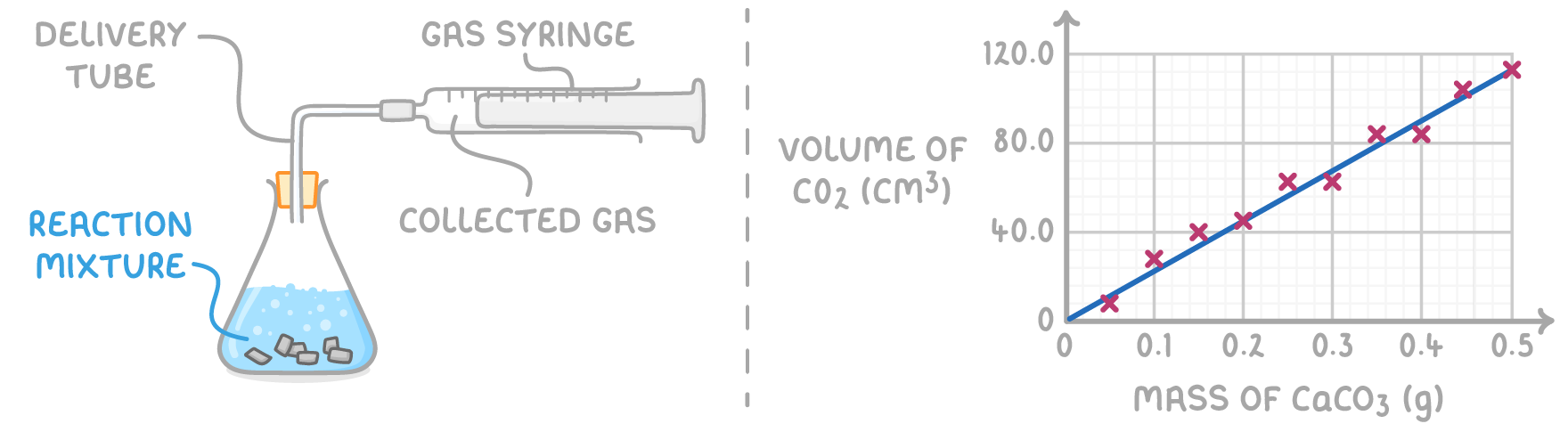
The steps to measure the molar volume of carbon dioxide are as follows:
- Add excess hydrochloric acid to a conical flask connected to a gas syringe.
- Add a known mass of calcium carbonate and allow the reaction to complete.
- Record the volume of carbon dioxide collected.
- Repeat using different calcium carbonate masses.
- Plot the volume of gas produced against the mass of CaCO3 used.
- Determine the volume of CO2 per mole of CaCO3 from the gradient.
This represents the molar volume of CO2 under the tested conditions.
Calculating gas volumes using molar volume
Molar volume and mole calculations can be combined to determine the volume of gas produced in a reaction.
Worked example 3 - Calculating gas volume
Calculate the volume of chlorine gas (Cl2) produced in dm3 when 30.0 g of manganese dioxide (MnO2) reacts with excess hydrochloric acid (HCl) at room temperature and pressure (r.t.p). The balanced equation is:
4HCl(aq) + MnO2(s) ➔ MnCl2(aq) + 2H2O(l) + Cl2(g)
Step 1: Calculate number of moles of MnO2
n =Mr m=86.930.0=0.345 mol
Step 2: Calculate number of moles of Cl2
Cl2 : MnO2 mole ratio = 1:1
Moles of Cl2 = 0.345 mol
Step 3: Rearrange equation
V = n x Vm
Step 4: Substitution and correct evaluation
V = 0.345 x 24 = 8.3 dm3
Worked example 4 - Calculate gas volumes using reaction stiochiometry
Calculate the total volume of gases produced in dm3 when 12.0 dm3 of ammonia (NH3) gas reacts with excess oxygen (O2) at room temperature and pressure (r.t.p) according to the balanced equation:
4NH3(g) + 5O2(g) ➔ 4NO(g) + 6H2O(g)
Step 1: Calculate number of moles of NH3
n =VmV=2412.0=0.50 mol
Step 2: Calculate total moles of gas produced
From the balanced equation, 4 mol of NH3 produces 4 (NO) + 6 (H2O) = 10 mol of gas in total.
0.50 mol of NH3 produces 0.50 x 410 = 1.25 mol of gas in total
Step 3: Rearrange equation
V = n x Vm
Step 4: Substitution and correct evaluation
V = 1.25 x 24 = 30 dm3