Properties of Alcohols
This lesson covers:
- The functional group and general formula of alcohols
- How alcohols are classified as primary, secondary or tertiary
- How alcohols form hydrogen bonds
- The physical properties of alcohols
Alcohols contain the hydroxyl functional group
Alcohols are organic compounds that contain a hydroxyl (-OH) functional group bonded to a carbon atom.
The general formula for alcohols is:
CnH2n+1OH
Where 'n' represents the number of carbon atoms.
Alcohols are named by replacing the ending "-e" of the corresponding alkane with "-ol", for example:
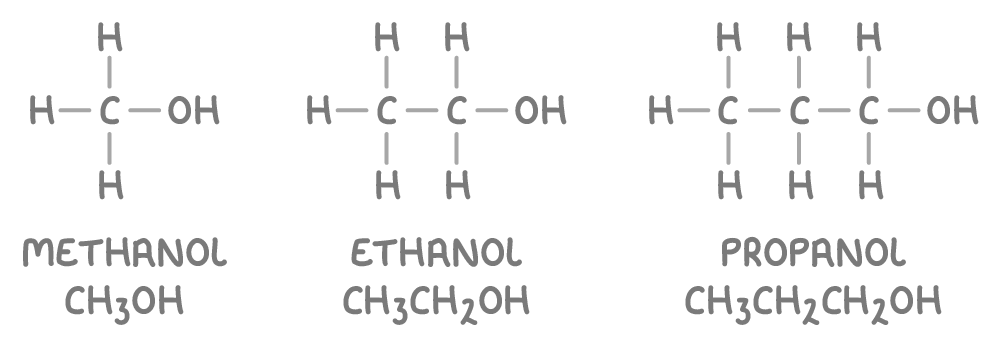
Where neccessary, a numerical prefix to the name is added to indicate the position of the hydroxyl group, for example, propan-1-ol.
Classification of alcohols
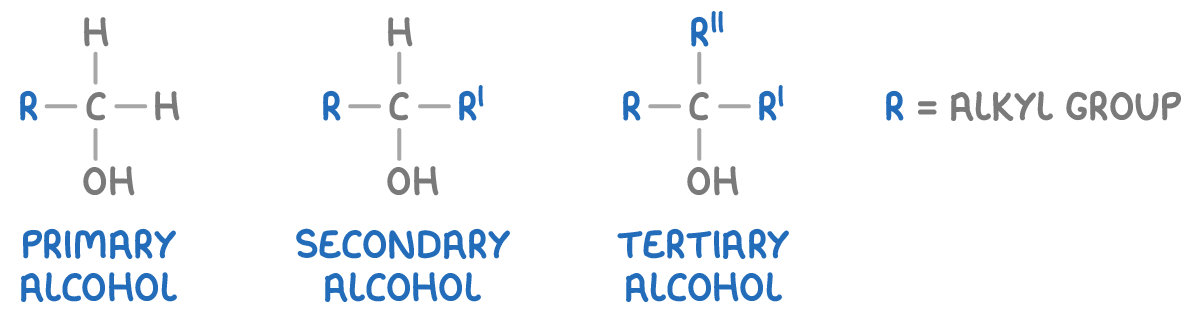
Alcohols are categorised into three types based on the carbon atom to which the hydroxyl group is attached:
- Primary alcohols - The -OH group is attached to a carbon atom that is bonded to only one alkyl group (or no alkyl groups).
- Secondary alcohols - The -OH group is attached to a carbon atom that is bonded to two alkyl groups.
- Tertiary alcohols - The -OH group is attached to a carbon atom that is bonded to three alkyl groups.
Alcohols form hydrogen bonds
The bond between oxygen and hydrogen in the hydroxyl group of alcohols is polar. This is due to oxygen being more electronegative than hydrogen, leading to an unequal sharing of electrons. As a result, the hydrogen atom acquires a partial positive charge (δ+), while the oxygen atom gains a partial negative charge (δ-).
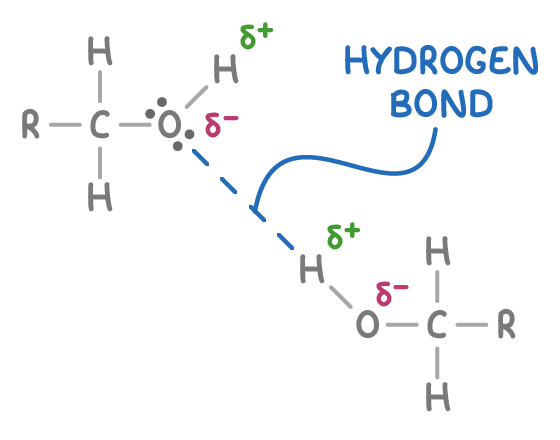
The partially positively charged hydrogen atom (δ+) can form intermolecular hydrogen bonds with the lone pairs of electrons on oxygen atoms found in other polar molecules, including water molecules or other alcohol molecules (as shown above).
Physical properties of alcohols
Alcohols have unique physical properties due to the ability of the hydroxyl (-OH) group to form hydrogen bonds.
- Solubility
- Alcohols with small carbon chains (methanol to propanol) dissolve in water because the hydroxyl group forms strong hydrogen bonds with water molecules.
- In contrast, alkanes are insoluble in water due to their lack of polar groups and inability to form hydrogen bonds.
- Larger alcohols have increased non-polar hydrocarbon regions that interfere with hydroxyl-water hydrogen bonding. So the solubility of alcohols in water decreases as their molecular size increases.
- Volatility
- The ability of alcohol molecules to hydrogen bond with each other leads to lower volatility (higher boiling points) in comparison to alkanes of similar molecular size.
- For example, ethanol is less volatile than ethane, despite having similar molecular masses.
- This is because the hydrogen bonds between alcohol molecules require more energy to break during the change of state from liquid to gas.