Carbon-carbon Bond Formation
This lesson covers:
- How nitriles are formed from haloalkanes and carbonyl compounds
- The mechanisms for nitrile formation
- The reduction and hydrolysis reactions of nitriles
- Alkylation and acylation reactions with benzene rings
Nitriles contain a C≡N functional group
Organic nitriles contain the nitrile functional group, C≡N.
Nitriles are useful intermediates in organic synthesis because:
- They can be easily converted into other functional groups like amines and carboxylic acids through reduction and hydrolysis.
- Reactions that form nitriles create new carbon–carbon bonds. Forming C-C bonds is vitally important in organic chemistry as it allows the synthesis of new compounds with longer carbon chains.
Some key organic synthesis reactions involving nitriles are discussed below.
Forming nitriles from halogenoalkanes
Nitriles can be synthesised through a nucleophilic substitution reaction between a halogenoalkane and a cyanide salt, like potassium cyanide (KCN), in ethanol. This reaction extends the carbon chain by 1 atom.
For example, bromoethane which contains 2 carbon atoms can be converted into propanenitrile which contains 3 carbon atoms:
CH3CH2Br + KCN ➔ CH3CH2CN + KBr
Mechanism of nitrile formation
The formation of nitriles from halogenoalkanes, such as bromoethane, involves a nucleophilic substitution mechanism:
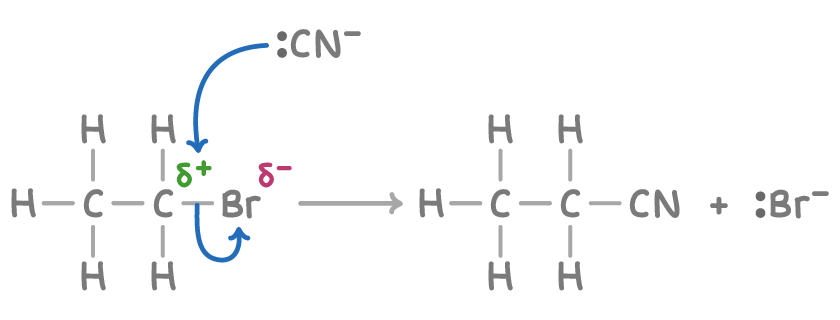
- The cyanide ion (CN-) acts as a nucleophile, attacking the carbon atom that is partially positive due to its attachment to electronegative bromine atom.
- This action breaks the carbon-bromine bond, substituting the halogen with a nitrile group and displacing a bromide ion.
Forming hydroxynitriles from carbonyl compounds
Aldehydes and ketones can react when heated with hydrogen cyanide (HCN) in a nucleophilic addition reaction to form a product known as a hydroxynitrile. This reaction also increases the carbon chain length.
For example, ethanal which contains 2 carbon atoms can be converted into 2-hydroxypropanenitrile which contains 3 carbon atoms:
CH3CHO + HCN ➔ CH3CH(OH)CN
Hydrogen cyanide is very hazardous to use directly, so it is often generated in situ by mixing sodium cyanide with sulfuric acid.
Mechanism of hydroxynitrile formation
The formation of hydroxynitriles from carbonyl compounds, such as ethanal, follows a nucleophilic addition mechanism:
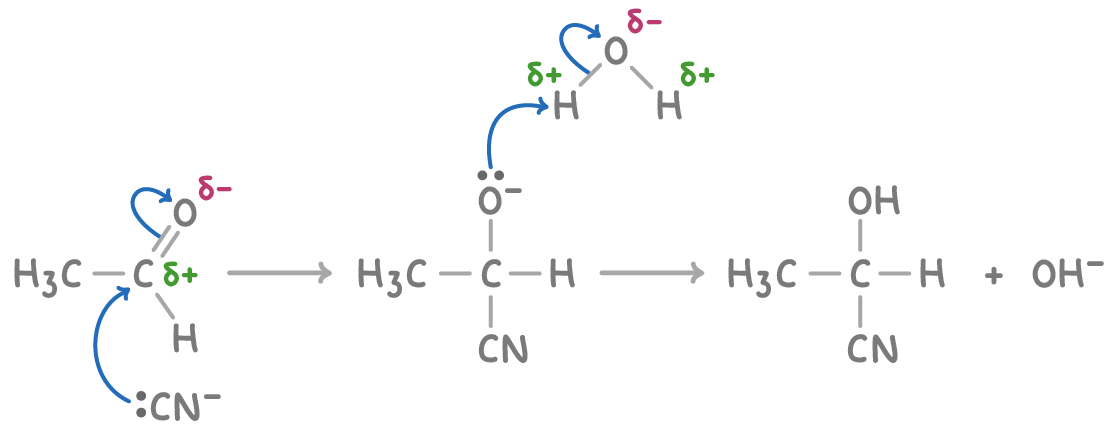
- The cyanide ion (CN-), acting as a nucleophile, attacks the partially positive carbon atom.
- A bond between carbon and cyanide forms, and an OH group is added.
- An H+ ion is lost, resulting in the hydroxynitrile product.
Nitriles are useful synthetic intermediates
Nitriles can be easily transformed into two other key functional groups: amines and carboxylic acids.
Reducing nitriles to primary amines
Catalytic hydrogenation of nitriles yields primary amines. This involves reacting the nitrile with hydrogen gas (H2) using a nickel catalyst.
For example, propanenitrile is reduced to propylamine:
CH3CH2C≡N + 2H2 ➔ CH3CH2CH2NH2
Here, the nitrile triple bond is reduced to a single N-H bond.
Hydrolysing nitriles to carboxylic acids
Hydrolysis of nitriles by heating with dilute aqueous acid converts the nitrile into a carboxylic acid through a nucleophilic addition-elimination mechanism.
For example, propanenitrile is hydrolysed to propanoic acid:
CH3CH2C≡N + 2H2O + HCl ➔ CH3CH2COOH + NH4Cl
Water molecules act as the nucleophile, adding across the triple bond. Ammonium chloride is a by-product, and a carboxyl group is introduced.
Forming carbon-carbon bonds with benzene
Alkylation and acylation reactions can introduce alkyl and acyl side chains onto a benzene ring, respectively.
Alkylation of benzene
Alkylation involves adding an alkyl group to a benzene ring using a halogenoalkane and a Friedel-Crafts catalyst, such as aluminium chloride (AlCl3).
For example, the ethylation of benzene using chloroethane is:
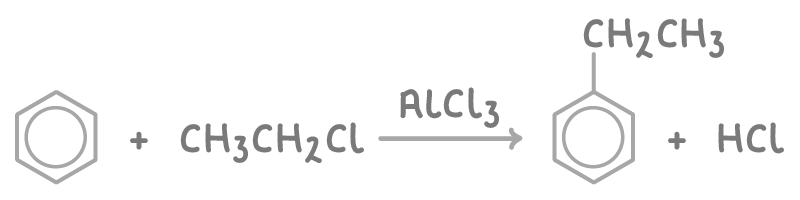
The products of alkylbenzene are valuable chemical feedstocks and provide substituents for further reactions on the benzene ring.
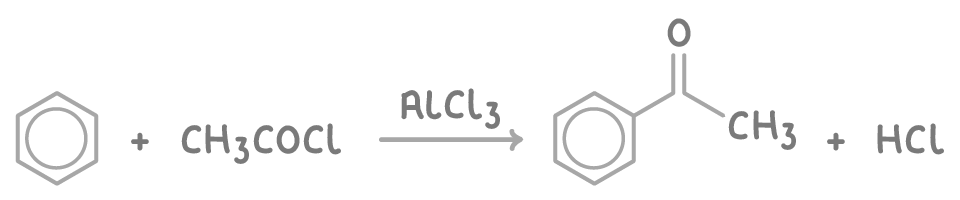
Acylation of benzene
Acylation introduces an acyl group (RCO-) onto a benzene ring using an acyl chloride and a Friedel-Crafts catalyst like aluminium chloride (AlCl3).
This process converts benzene into a ketone, adding an additional reactive functional group for further reactions.
For example, the acylation of benzene using ethanoyl chloride is: