Amides
This lesson covers:
- Amides as carboxylic acid derivatives
- Making amides from acyl chlorides
Amides are carboxylic acid derivatives
Amides are organic compounds that contain the functional group –CONH2. They are derived from carboxylic acids by replacing the hydroxyl group with an amino group.
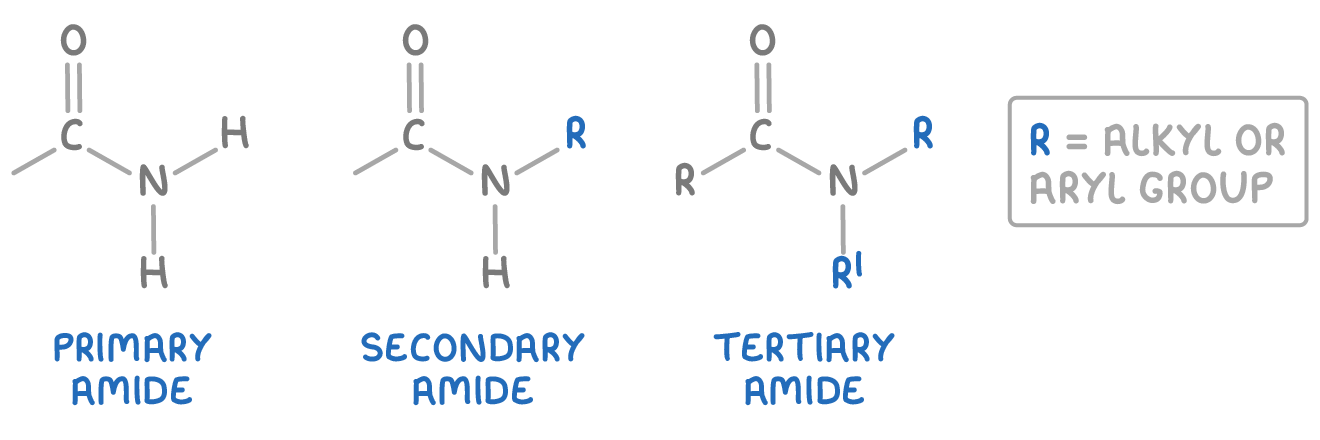
There are three main types of amide you should be familiar with:
- Primary amides - These are amides where the nitrogen atom is bonded to one carbonyl group (C=O) and two hydrogen atoms.
- Secondary amides - These are amides where the nitrogen atom is bonded to one carbonyl group (C=O), one hydrogen atom and one alkyl or aryl group.
- Tertiary amides - These are amides where the nitrogen atom is bonded to one carbonyl group (C=O), and two alkyl or aryl groups.
Making amides from acyl chlorides
Amides can be synthesised through the reaction of acyl chlorides with concentrated ammonia or primary amines, typically occurring at room temperature.
With ammonia:
The reaction between acyl chlorides and ammonia produces primary amides.
For example, ethanoyl chloride reacts with ammonia to give ethanamide and HCl:
CH3COCl + NH3 ➔ CH3CONH2 + HCl
With amines:
The reaction between acyl chlorides and primary amines produces secondary amides, also known as N-substituted amides.
For example, ethanoyl chloride reacts with methylamine to give N-methylethanamide and HCl:
CH3COCl + CH3NH2 ➔ CH3CONHCH3 + HCl
In these reactions, the produced HCl typically reacts with any excess ammonia or amine to form ammonium salts.