Properties of the Alkenes
This lesson covers:
- The general formula and bonding of alkenes
- The components of a carbon-carbon double bond
- The trigonal planar geometry of alkenes
- Why alkenes are more reactive than alkanes
Alkenes have the general formula CnH2n
Alkenes are a class of hydrocarbons containing carbon-carbon double bonds. Their general formula is CnH2n, where n represents the number of carbon atoms.
Key features of alkenes:
- They consist of carbon and hydrogen atoms (hydrocarbons)
- Each alkene molecule contains at least one carbon-carbon double bond
- The double bond makes them "unsaturated", enabling them to participate in addition reactions.
Examples of alkenes:
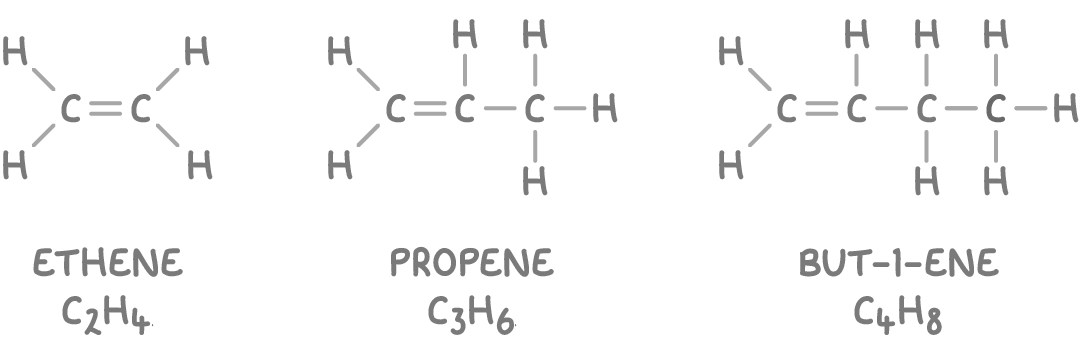
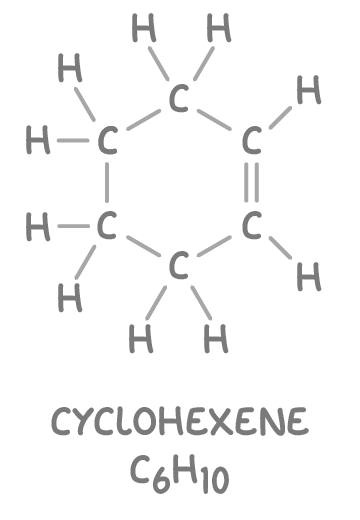
Note that cyclic alkenes such as cyclohexene (C6H10) have two fewer hydrogen atoms compared to acyclic alkenes with an equivalent number of carbon atoms.
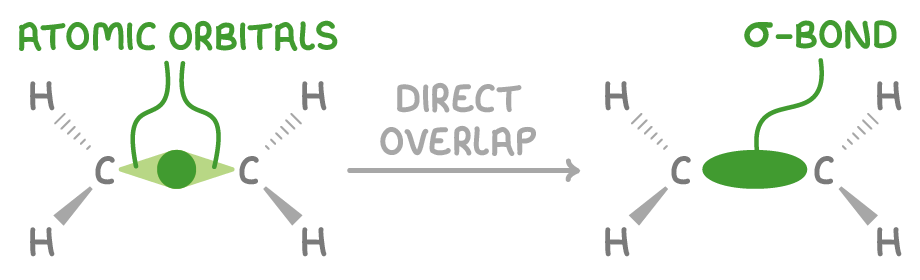
A double bond consists of a sigma bond and a pi bond
The carbon-carbon double bond in alkenes comprises:
1. A sigma (σ) bond:
- This is a strong bond formed by the head-on overlap of carbon s orbitals.
- It has a high electron density situated directly between the nuclei.
- It is very strong, possessing the highest bond enthalpy among single bonds.
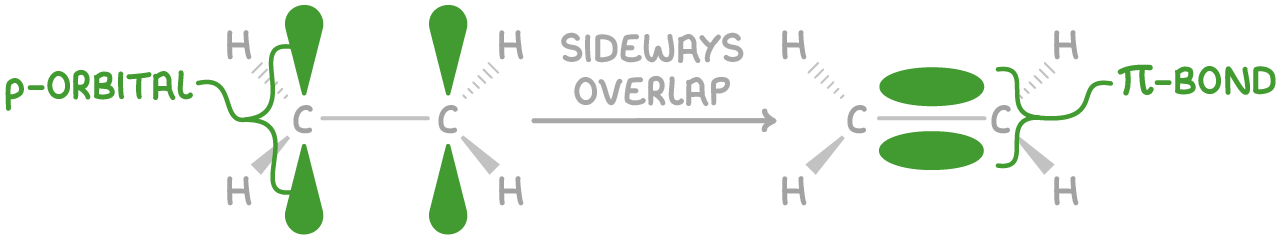
2. A pi (π) bond:
- This bond is created by the sideways overlap of adjacent p orbitals.
- Its electron density is distributed above and below the bond axis.
- It has a lower electron density and bond enthalpy compared to the sigma bond.
- The presence of the pi bond restricts rotation around the C=C bond, as rotation would disrupt the parallel overlap of the p orbitals, leading to a significant increase in energy.
Trigonal planar geometry of carbon in alkenes
In alkenes, each carbon atom within the C=C double bond has:
- Three regions of electron density.
- These regions are arranged in a trigonal planar shape due to equal repulsion between them.
- The bond angles around the carbon atoms are approximately 120°.
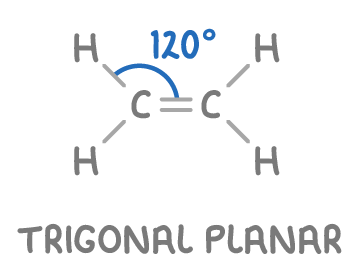
An example of this geometry is seen in ethene (C2H4), where each carbon in the double bond displays trigonal planar geometry.
Alkenes are more reactive than alkanes due to their pi bond
Alkanes contain only strong sigma (σ) bonds between carbon atoms.
These nonpolar sigma bonds contribute to low reactivity in alkanes:
- Their high bond enthalpy makes alkane bonds difficult break.
- They do not attract reactive nucleophiles and electrophiles.
By contrast, alkenes contain a pi (π) bond in their C=C double bond.
Three key features make this pi bond more reactive:
- High electron density - Makes pi electrons highly accessible for reactions.
- Protruding shape - Allows electrophiles to readily attack pi electrons.
- Lower bond enthalpy - Requires less energy to break than sigma bonds.
These structural features of the pi bond give alkenes vastly greater reactivity over alkanes. The pi bond enables alkenes to readily undergo addition reactions across the C=C double bond.