Neutralisation
This lesson covers:
- Using a pH meter to measure pH
- The shape of pH curves for different combinations of acids and bases
- Choosing an appropriate indicator for a titration
- Calculating concentrations using titration data
Measuring pH with a pH meter
A pH meter is an electronic device used to find out the pH of a solution. It has a probe with a sensitive bulb at the bottom, which is dipped into the solution, and a digital display that shows the pH reading.
To get accurate measurements, the pH meter must be calibrated before use:
- Put the probe in distilled water and adjust the reading to 7.0.
- Do the same with standard solutions of pH 4 and pH 10, rinsing the probe with distilled water between each reading.
When measuring the pH of a solution:
- Dip the probe in the liquid and wait for the reading to stabilise.
- Write down the result and rinse the probe with distilled water after each measurement.
Plotting pH curves for acid-base titrations
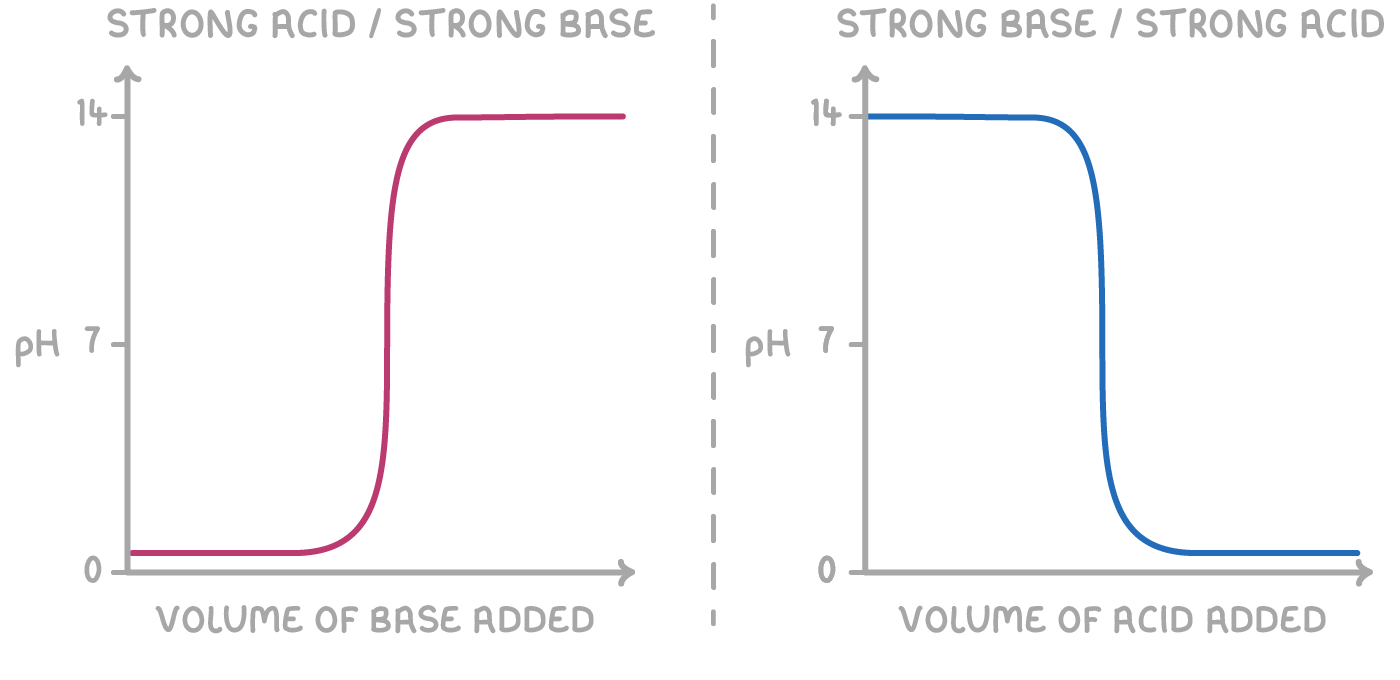
pH curves are graphs that show how the pH changes during a titration, plotting the pH of the mixture against the volume of acid or base added. The curve's shape depends on the strengths of the acid and base involved.

The shape of each graph is explained by considering:
1. Initial pH - The starting pH depends on the acid's strength. Strong acid titrations begin at a lower pH than weak acid ones.
2. Early stages - At the start, adding small amounts of base barely affects the solution's pH.
3. Equivalence point - The nearly straight part of the graph represents the equivalence point, where the moles of acid and base are stochiometrically equivalent, resulting in complete neutralisation. Here, a tiny amount of base causes a sudden, big change in pH.
- For a strong acid/strong base titration, a small amount of base leads to a rapid pH change.
- For a strong acid/weak base titration, more weak base is needed to change the pH, and the change is less noticeable.
- For a weak acid/strong base titration, less strong base is needed to cause a big pH change.
- For a weak acid/weak base titration, there's no sharp pH change at the equivalence point, making it hard to spot the exact end point using an indicator. The endpoint is the point at which the reaction between the acid and base is complete, and it coincides with the equivalence point.
4. Final pH - The pH at the titration's end depends on the base's strength. The stronger the base, the higher the final pH.
For titrations of a base with an acid, the pH curves have the same shapes but flip vertically, with pH decreasing as more acid is added.
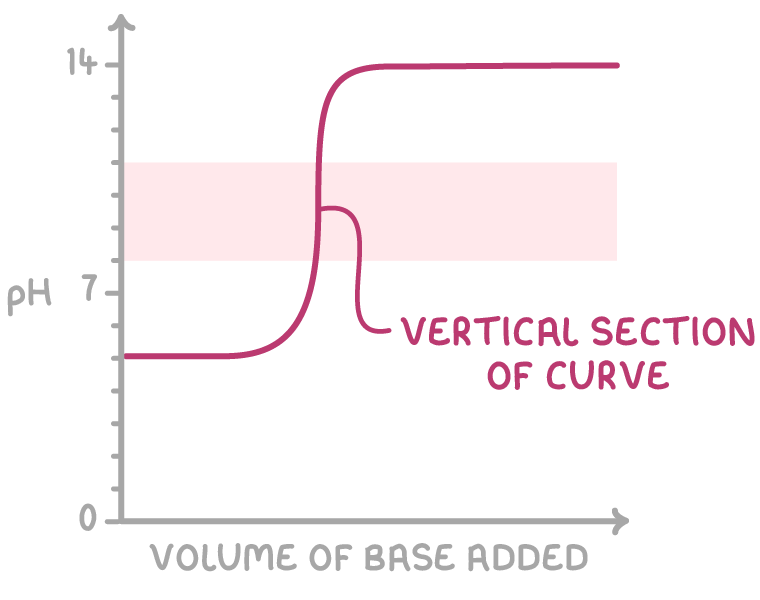
Selecting indicators based on pH curves
Indicators are weak acids that change colour over a specific pH range, allowing them to be used to determine the end point of a titration. The choice of indicator depends on the pH curve of the titration. For accurate results, the indicator should undergo a sharp and distinct colour change entirely within the steep vertical section of the pH curve around the equivalence point.
The colour change in indicators occurs because they have differently coloured conjugate acid-base pairs. As the pH changes during a titration, the equilibrium concentrations of the conjugate pairs also change, resulting in a colour change depending on whether the indicator is mainly protonated or deprotonated.
Consider the equilibrium for the acid-base indicator methyl orange:
Methyl orange-H ⇌ methyl orange- + H+
(red) (yellow)
Upon addition of a basic solution containing OH- ions:
- OH- ions from the base react with H+ ions in the methyl orange indicator.
- The position of equilibrium shifts to the right.
- The colour of the solution changes to yellow, the colour of methyl orange-.
Upon addition of an acidic solution containing H+ ions:
- H+ ions from the acid react with methyl orange- ions from the indicator.
- The position of equilibrium shifts to the left.
- The colour of the solution changes to red, the colour of methyl orange-H.
At the end point of a titration, the solution contains equal concentrations of methyl orange-H and methyl orange-, so the colour of the indicator will be orange, in between the red and yellow colours of methyl orange-H and methyl orange-, respectively.
The following table summarises the properties and suitable titrations for methyl orange and phenolphthalein:
| Name of indicator | Colour at low pH | Colour at high pH | pH range | Titrations suitable for |
|---|---|---|---|---|
| Methyl orange | Red | Yellow | 3.1 – 4.4 | Strong acid/strong base / Strong acid/weak base |
| Phenolphthalein | Colourless | Pink | 8.3 – 10 | Strong acid/strong base / Weak acid/strong base |
For weak acid/weak base titrations, no suitable indicators exist because of the gradual pH change at the equivalence point. Instead, a pH meter should be used to accurately find the titration's end point.
Calculating concentrations using titration data
To calculate the concentration of an acid or base from titration results:
- Accurately measure the neutralisation volume (usually to the nearest 0.05 cm3).
- Repeat the titration at least three times and calculate the average.
- Ignore any results that don't match; all results should be concordant (within 0.10 cm3 of each other).
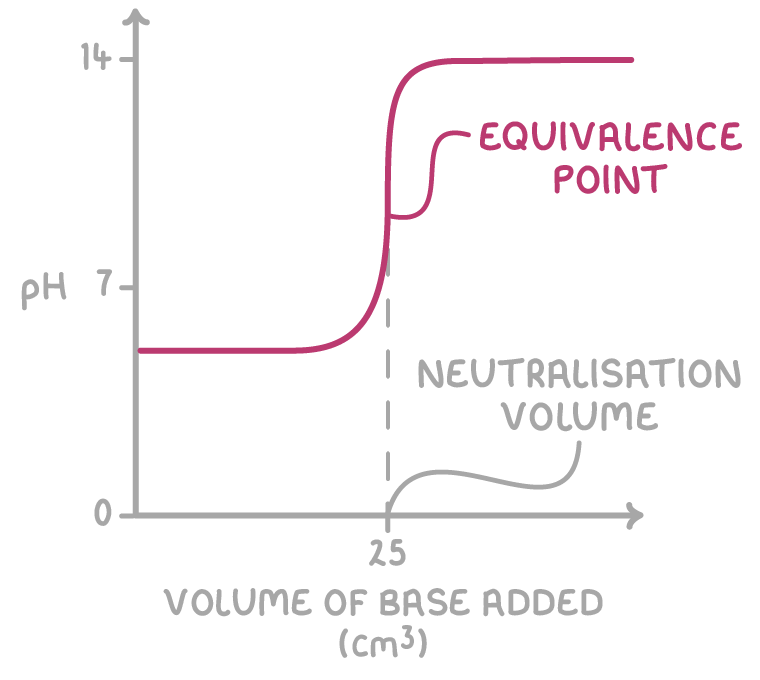
Use a pH meter to pinpoint the equivalence point by locating the midpoint of the vertical section on the pH curve and drawing a line down to the x-axis. The volume at this point shows the amount of acid or base needed for neutralisation.
Worked example 1 - Calculating the concentration of a sodium hydroxide solution
30.0 cm3 of 1.00 mol dm-3 HCl was required to neutralise 50.0 cm3 of NaOH solution:
HCl(aq) + NaOH(aq) ➔ NaCl(aq) + H2O(l)
Calculate the concentration of the sodium hydroxide solution.
Step 1: Conversion from cm3 into dm3
To convert from cm3 into dm3, divide by 1,000
30.0 cm3 = 0.0300 dm3
50.0 cm3 = 0.0500 dm3
Step 2: Calculate number of moles of HCl
n = c × V=1.00×0.0300=0.0300 mol
Step 3: Calculate number of moles of NaOH neutralised
NaOH : HCl mole ratio = 1:1
Moles of NaOH = 0.0300 mol.
Step 4: Calculate concentration of NaOH
c =Vn=0.05000.0300=0.600 mol dm−3
Worked example 2 - Calculating the concentration of ethanedioic acid
25.0 cm3 of ethanedioic acid, C2H2O4, was completely neutralised by 30.0 cm3 of 0.150 mol dm-3 KOH solution:
(COOH)2(aq) + 2KOH(aq) ➔ (COOK)2 + 2H2O(l)
Calculate the concentration of the ethanedioic acid.
Step 1: Conversion from cm3 into dm3
To convert from cm3 into dm3, divide by 1,000
25.0 cm3 = 0.0250 dm3
30.0 cm3 = 0.0300 dm3
Step 2: Calculate number of moles of KOH
n = c × V=0.150×0.0300=4.50×10−3 mol
Step 3: Calculate number of moles of (COOH)2 neutralised
(COOH)2 : KOH mole ratio = 1:2
Moles of (COOH)2 = 2(4.50×10−3)=2.25×10−3 mol
Step 4: Calculate concentration of (COOH)2
c =Vn=0.0250(2.25×10−3)=9.00×10−2 mol dm−3