Electron Structure
This lesson covers:
- The arrangement of electrons in shells, sub-shells, and orbitals
- The shapes of s and p orbitals
- Different ways to represent electron configuration
- How to determine electron configurations
Electron shells contain sub-shells and orbitals
In the modern model of the atom, electrons are found in specific energy levels known as shells surrounding the nucleus.
- Shells that are further from the nucleus hold electrons with higher energy compared to those closer.
- Each shell is defined by a principal quantum number (n = 1, 2, 3...), indicating its relative distance from the nucleus.
- These shells are further divided into sub-shells, which have distinct energy levels and are labelled as s, p, d, and f.
- Within sub-shells, electrons are located in orbitals, which are regions with a high probability of finding an electron. An orbital is defined as a region around the nucleus that can accommodate up to two electrons with opposite spins.
The capacity of each sub-shell type to hold electrons is detailed in the following table:
| Sub-shell | Number of orbitals | Maximum number of electrons |
|---|---|---|
| s | 1 | 2 |
| p | 3 | 6 |
| d | 5 | 10 |
| f | 7 | 14 |
The distribution of sub-shells across the first four shells is as follows:
| Shell | Sub-shells | Total number of electrons |
|---|---|---|
| 1 | 1s | 2 |
| 2 | 2s, 2p | 8 |
| 3 | 3s, 3p, 3d | 18 |
| 4 | 4s, 4p, 4d, 4f | 32 |
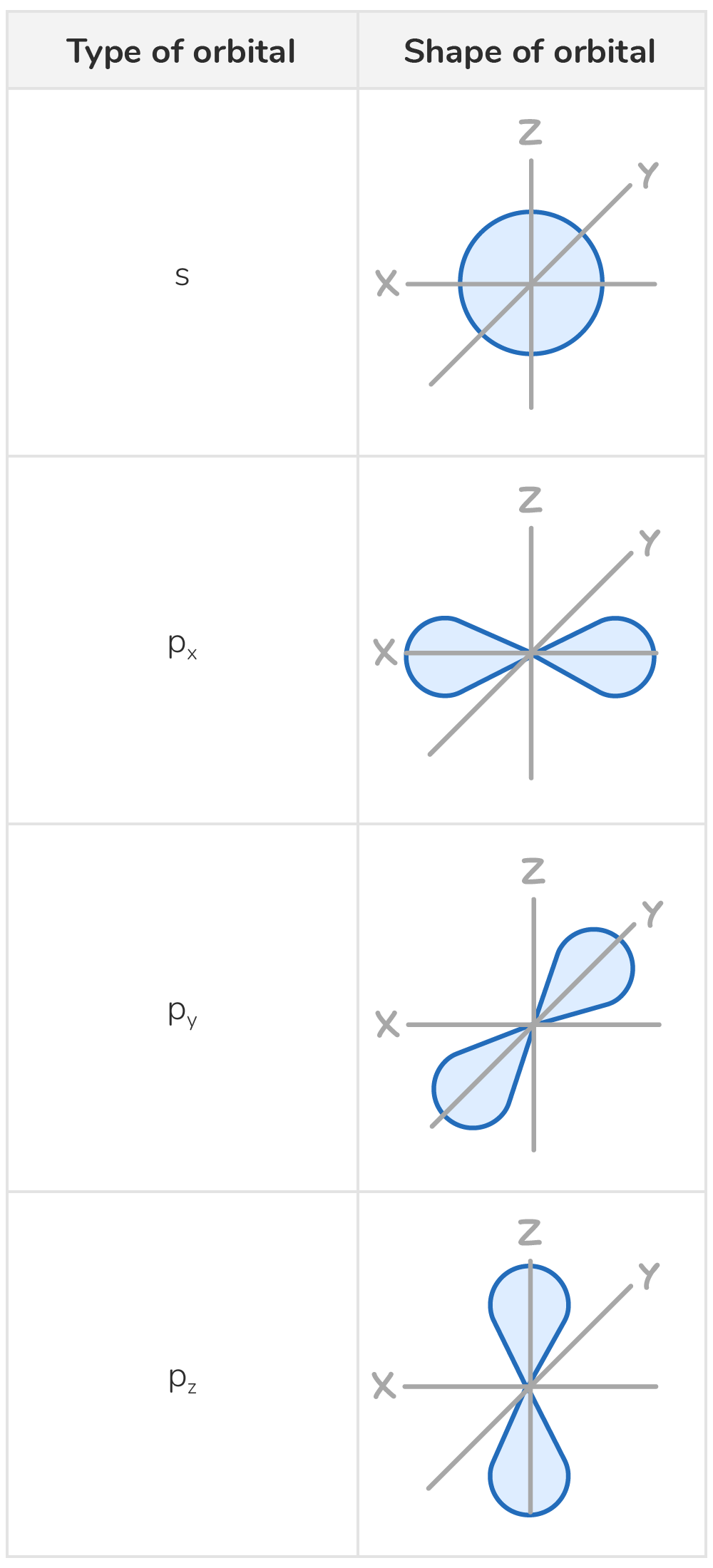
Orbitals have characteristic shapes
Orbitals, the regions in which electrons are most likely to be found, have unique shapes:
- Orbitals within the same sub-shell are of equal energy.
- Each orbital can hold two electrons, which must have opposite spins (called spin-pairing).
- s orbitals are spherical in shape
- p orbitals are dumbbell-shaped. The three p orbitals are oriented at right angles to each other.
Representing electron configurations
The arrangement of electrons in an atom is called its electron configuration. This can be depicted in multiple ways:
1. Sub-shell notation
This method uses superscripts to indicate the number of electrons within each sub-shell.
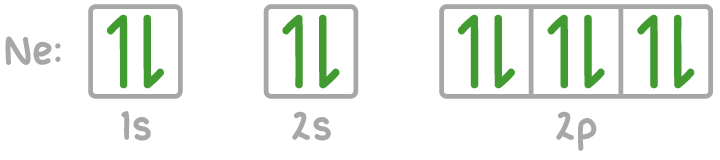
For example, the electron configuration of neon (10 electrons) is 1s² 2s² 2p⁶.
2. Electrons-in-boxes notation
- Orbitals are depicted as boxes, with electrons shown as arrows.
- Oppositely directed arrows represent electrons with opposite spins.
- Electron pairing within orbitals occurs only with opposite spins.
For example, the electron configuration of neon is shown below.
Electron configurations represent the most stable arrangement
Electron configurations are arranged to minimise the overall energy of the atom or ion.
This lowest energy arrangement corresponds to the most stable electronic structure.
To deduce an atom's electron configuration, follow these guidelines:
- Electrons fill the lowest energy orbitals first.
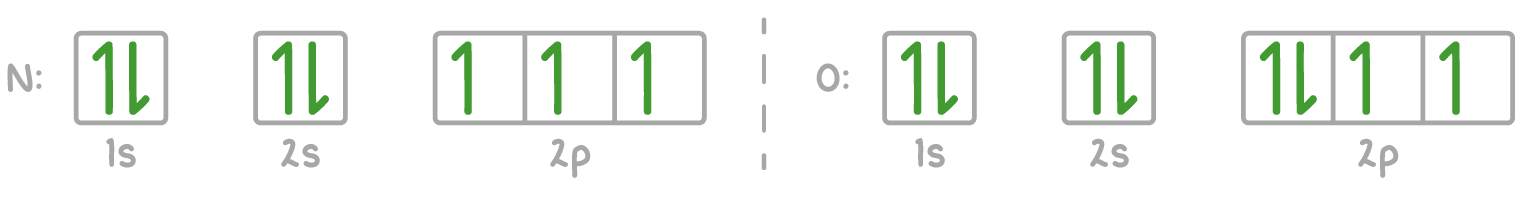
For example, calcium's electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s², which can be represented using the electrons-in-boxes notation as follows:

The 4s sub-shell is filled before the 3d sub-shell because the 4s orbital has a lower energy than the 3d orbitals in neutral atoms.
2. Electrons first occupy orbitals of equal energy singly before pairing up.
3. When two electrons occupy the same orbital, they must have opposite spins (up and down) to minimise electron-electron repulsion.
4. For ions in the s and p blocks, electrons are added to or removed from the highest occupied sub-shell, e.g.:
- The electronic configuration of Mg²⁺ is 1s² 2s² 2p⁶.
- The electronic configuration of Cl⁻ is 1s² 2s² 2p⁶ 3s² 3p⁶.
Note: Noble gas notation can simplify representations, using square brackets for the electron configuration of the preceding noble gas.
For example, calcium's configuration (1s² 2s² 2p⁶ 3s² 3p⁶ 4s²) is abbreviated as [Ar] 4s², where [Ar] represents 1s² 2s² 2p⁶ 3s² 3p⁶.