Storage and Fuel Cells
This lesson covers:
- How energy storage cells work
- How to calculate the cell voltage of an energy storage cell
- How fuel cells generate electricity
- The advantages and disadvantages of electrochemical cells
Energy storage cells function like electrochemical cells
Energy storage cells, also known as batteries, operate based on the principles of electrochemical cells. The key principle underpinning their function is that the two electrodes have different electrode potentials. This potential difference drives the cell reaction, allowing the battery to generate electricity.
The voltage that these cells produce can be calculated by looking at the electrode potentials of the substances inside the cell.
You won't need to memorise specific E⦵ values for the reactions, but you might need to calculate the cell potential or voltage for a particular cell. Let's go through an example to show you how.
Worked example 1 - Calculating the cell voltage of a lead-acid storage cell
Calculate the cell voltage produced by a lead-acid storage cell, which has a lead (Pb) anode, a lead dioxide (PbO2) cathode, and a sulfuric acid electrolyte.
| Half equation | E⦵ (V) |
|---|---|
| Pb2+(aq) + 2e- ⇌ Pb(s) | -0.13 |
| PbO2(s) + 4H+(aq) + SO42-(aq) + 2e- ⇌ PbSO4(s) + 2H2O(l) | +1.69 |
Step 1: Identify oxidation and reduction half-reactions
The Pb2+ half-reaction proceeds in the oxidation direction (Pb(s) ➔ Pb2+(aq) + 2e-), as indicated by its lower (i.e. negative) E⦵ value relative to the half-reaction of PbO2.
The PbO2 half-reaction proceeds in the reduction direction (PbO2(s) + 4H+(aq) + SO42-(aq) + 2e- ➔ PbSO4(s) + 2H2O(l)), as indicated by its higher (i.e. positive) E⦵ value.
Step 2: Equation
Ecell⊖=Ereduced⊖−Eoxidised⊖
Step 4: Substitution and correct evaluation
Ecell⊖=+1.69−(−0.13)=+1.82 V
Therefore, the cell voltage of the lead-acid battery is 1.82 V.
Fuel cells generate electricity by continuously reacting a fuel with oxygen
Fuel cells, unlike energy storage cells (batteries) that hold a finite amount of chemical energy, can generate electricity continuously as long as fuel and oxygen are provided.
Hydrogen fuel cells are seen as a promising power source for electric vehicles, providing an alternative to traditional internal combustion engines and batteries. In a hydrogen fuel cell, hydrogen (the fuel) and oxygen react to produce electricity, with water being the only by-product.
The process includes the following steps:
- Hydrogen oxidation at the anode - At the anode, hydrogen (H2) is split into protons (H+) and electrons (e-) with the help of a platinum catalyst.
- Proton migration through the electrolyte - The protons (H+) move through the polymer electrolyte membrane, which only allows protons to pass. This forces the electrons (e-) to travel through an external circuit to get to the cathode.
- Electron flow through the external circuit - The electrons flowing through the external circuit generate an electric current that can power devices.
- Oxygen reduction at the cathode - Oxygen (O2) at the cathode combines with the protons (H+) from the anode and the electrons (e-) from the circuit to produce water (H2O), the only waste product.
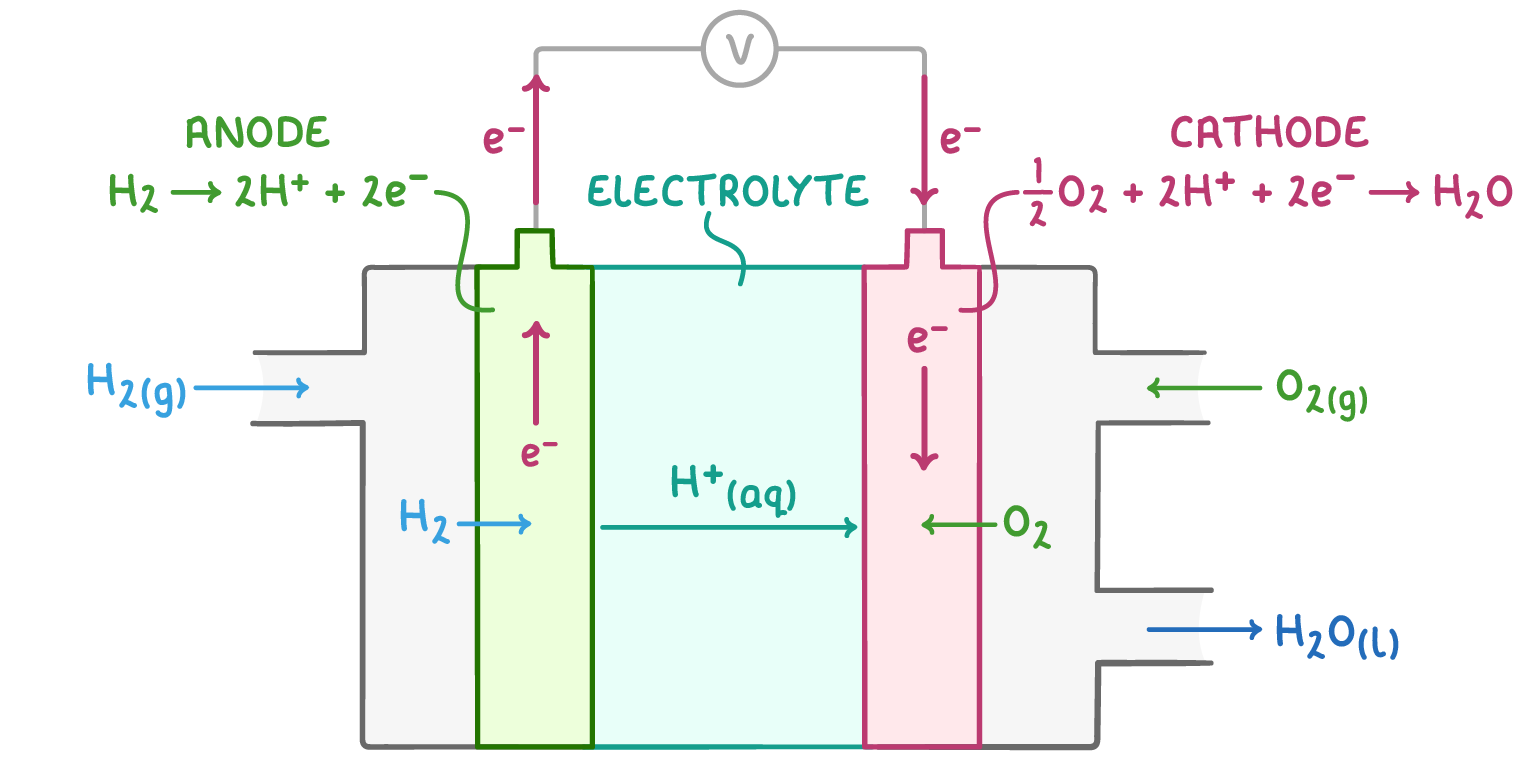
The overall reaction in the fuel cell is the formation of water from hydrogen and oxygen:
2H2(g) + O2(g) ➔ 2H2O(l)
The specific reactions at each electrode are:
- Negative electrode: H2(g) ➔ 2H+(aq) + 2e-
- Positive electrode: 1⁄2O2(g) + 2H+(aq) + 2e- ➔ H2O(l)
As hydrogen and oxygen are continuously supplied, the fuel cell will keep generating electricity. This is different from a battery, which needs recharging after its stored chemical energy is used up, making hydrogen fuel cells an exciting option for electric vehicles.
Advantages and disadvantages of electrochemical cells
Electrochemical cells offer several benefits compared to traditional combustion engines:
1. Greater efficiency - They convert a larger proportion of their available energy into electrical energy, whereas combustion engines lose much energy as heat.
2. Reduced emissions - Electrochemical cells produce significantly less pollution, such as CO2, compared to combustion engines.
3. Clean by-products - Hydrogen fuel cells, for instance, only produce water as a waste product.
However, there are also drawbacks to using electrochemical cells for energy storage and generation:
1. Toxic materials - Their production involves toxic chemicals that must be disposed of properly at the end of the cell's life to avoid environmental damage.
2. Flammability risks - The chemicals used are often highly flammable. For example, lithium, used in rechargeable batteries, is highly reactive and poses a safety risk if the cell overheats and catches fire.