Chirality
This lesson covers:
- What optical isomers are
- How to identify chiral centres and optical isomers
Optical isomers are mirror images
Optical isomerism is a type of stereoisomerism where isomers have the same structural formula but different arrangements of atoms in space.
A chiral carbon is a carbon with four different groups attached to it. When the groups around a chiral carbon are arranged differently, it results in two non-superimposable mirror image structures called optical isomers.
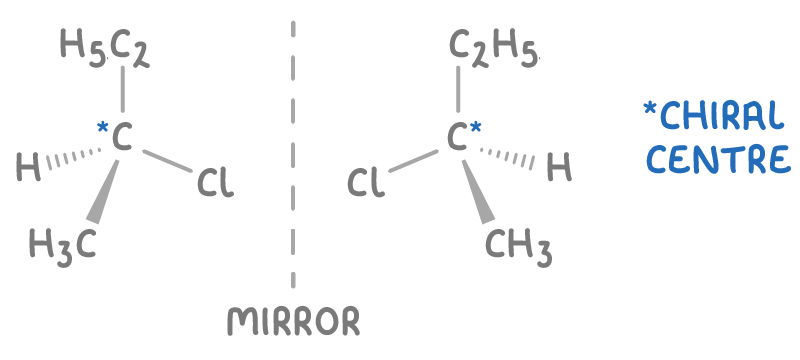
For instance, 2-chlorobutane has a chiral centre on its second carbon, bonded to a chlorine atom, a methyl group, an ethyl group, and a hydrogen atom, resulting in two optical isomers.
To identify and draw optical isomers, follow these steps:
- Identify the chiral carbon - Carefully draw all hydrogen atoms to clearly identify each attachment. Look for the carbon atom connected to four different groups.
- Sketch the optical isomers - Illustrate one enantiomer with its groups arranged tetrahedrally around the chiral carbon. Next, draw its mirror image.
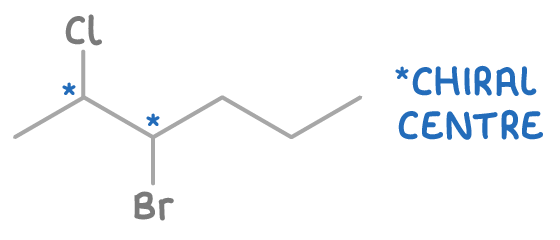
Molecules with multiple chiral centres can have more than two optical isomers, increasing the complexity and number of possible isomers.
For instance, 3-bromo-2-chloropentane has two chiral carbons and hence four optical isomers: