Catalysts
This lesson covers:
- What catalysts are and how they work
- Types of catalysts (homogeneous and heterogeneous)
- Applications of catalysts (industrial uses and environmental sustainability)
Definition of a catalyst
A catalyst is a substance that increases the rate of a chemical reaction. It does this by providing an alternative route for the reaction that has a lower activation energy. Importantly, the catalyst itself is not consumed in the reaction; it temporarily participates in the reaction but is chemically regenerated at the end.
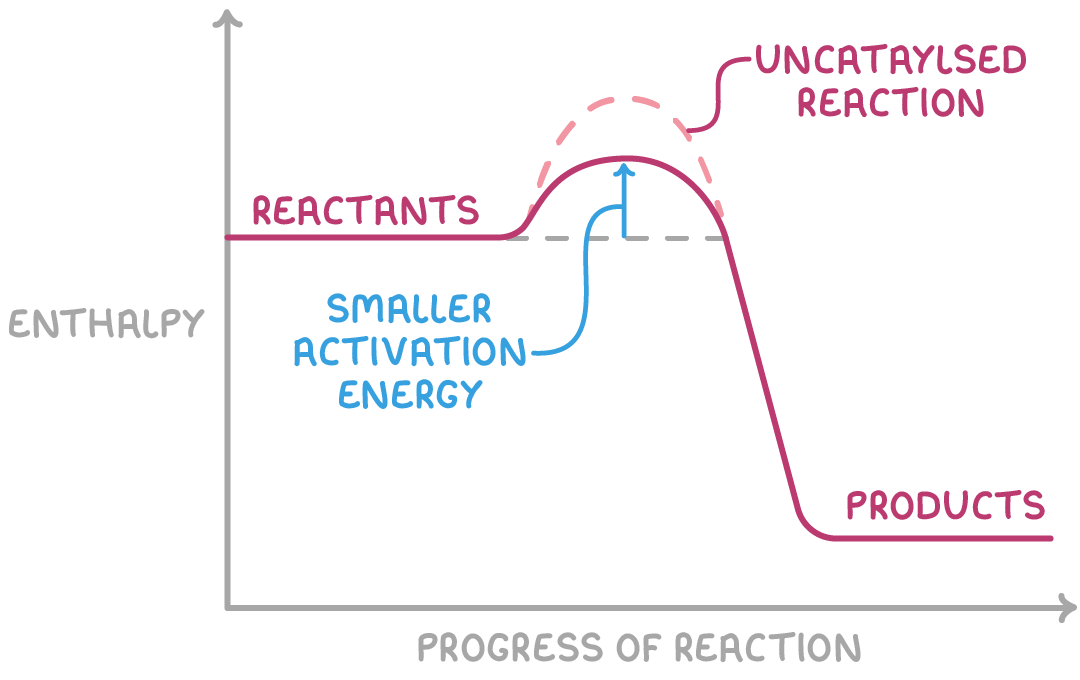
The enthalpy profile diagram above illustrates this concept by showing that the activation energy of the catalysed reaction pathway is lower than that of the uncatalysed reaction pathway.
How catalysts work
Catalysts are specific - they usually work only for particular reactions by interacting with specific reactants.
They provide an alternative pathway for the reaction which has a lower activation energy. This lower energy requirement means a larger number of reactant particles have sufficient energy to undergo the reaction.
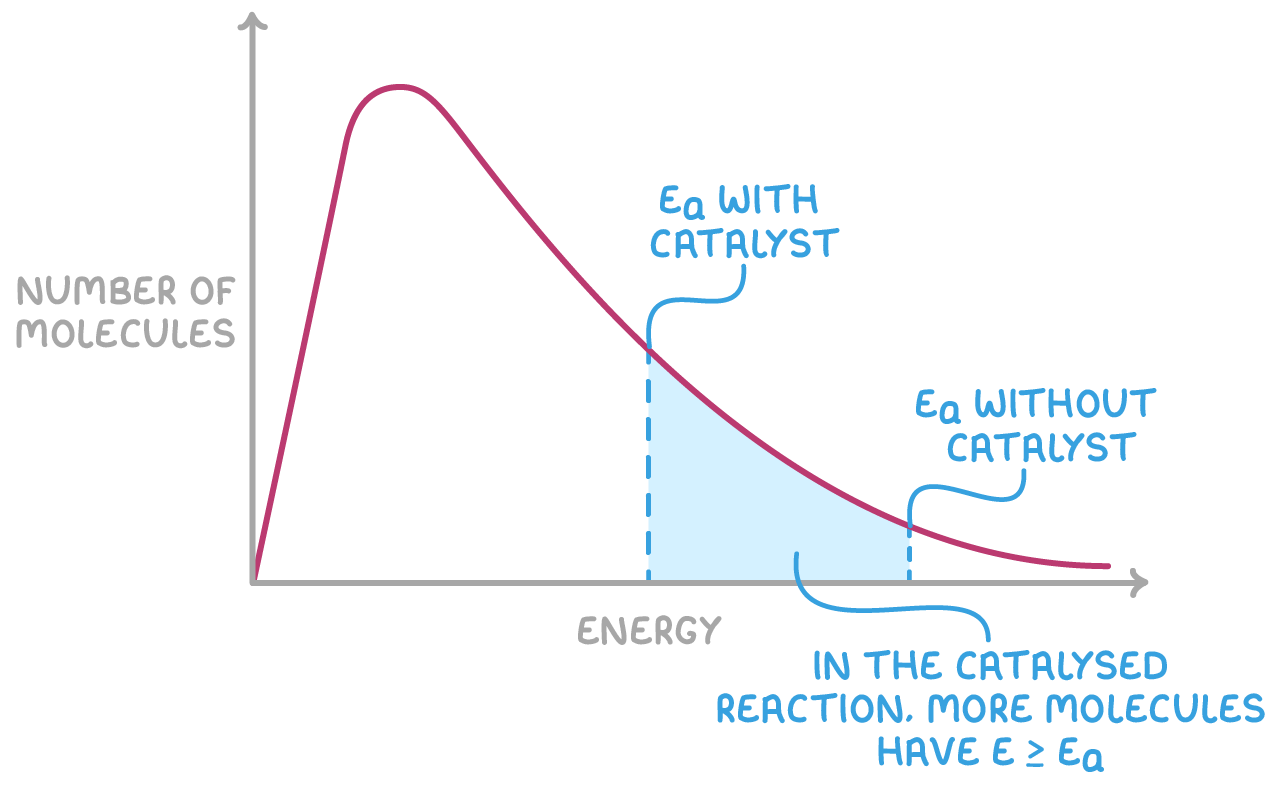
The Maxwell-Boltzmann distribution shows that, at a given temperature, the energies of gas particles are spread over a wide range. By lowering the activation energy, a catalyst increases the fraction of particles that have enough energy to react, thereby increasing the rate of the reaction.
Types of catalysts
There are two primary types of catalysts:
- Heterogeneous catalysts - These are in a different phase from the reactants.
- For example, in the Haber process, solid iron is used as a catalyst for gaseous nitrogen and hydrogen.
- The reaction takes place on the surface of the catalyst, so increasing the surface area can increase the rate of reaction.
- Homogeneous catalysts - These are in the same phase as the reactants.
- Typically, these are aqueous catalysts used in reactions involving aqueous solutions.
- An example is sulfuric acid serving as a catalyst in the reaction between aqueous hydrogen peroxide and aqueous potassium iodide, where it works by forming intermediate species that lead to the final products.
Benefits of catalysts
Economic benefits
Catalysts bring about significant economic advantages:
- They speed up production, allowing more products to be manufactured and sold.
- They enable reactions to occur at lower temperatures and pressures, reducing energy consumption.
- They improve atom economy, which minimises material wastage.
An example is the use of zeolite catalysts in developing lower-temperature processes for cracking hydrocarbons, producing more gasoline fuel from each barrel of oil.
Sustainability benefits
Catalysts contribute to more sustainable chemical processes:
- Reactions requiring lower temperatures result in reduced emissions of greenhouse gases, such as CO2.
- They allow for reactions with better atom economy, cutting down on the waste produced.
- Catalysts can accelerate the breakdown of pollutants from industrial processes.
A notable application is the use of platinum catalysts in vehicle catalytic converters, which accelerate the breakdown of harmful emissions like carbon monoxide and nitrogen oxides into less harmful substances, significantly reducing air pollution from vehicles.