Stereoisomerism in Complex Ions
This lesson covers:
- What stereoisomerism is
- Optical isomerism in complex ions
- Cis-trans isomerism in complex ions
- The role of cis-platin as an anti-cancer drug
Stereoisomers and complex ions
Stereoisomers are compounds with the same structural formula but different spatial arrangements of their atoms.
Complex ions can exhibit stereoisomerism because the ligands arranged around the central metal ion can be positioned in different ways.
Optical isomers in complex ions
Optical isomerism is a type of stereoisomerism where molecules exist as non-superimposable mirror images, known as enantiomers.
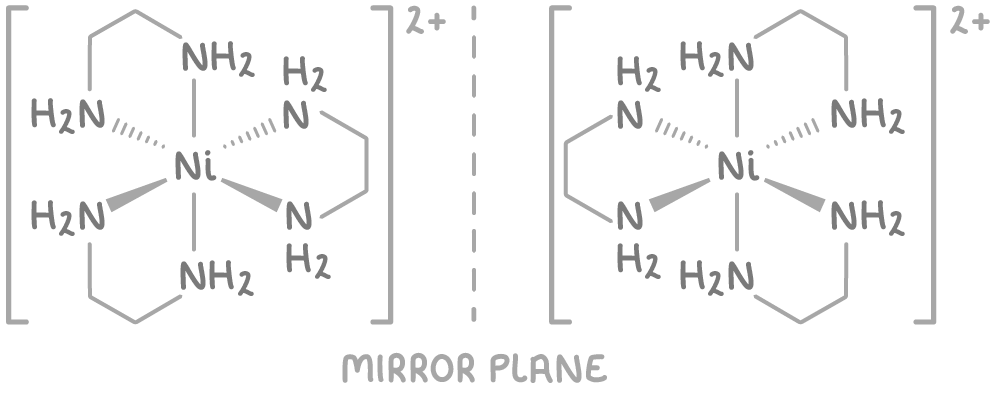
Optical isomerism occurs in octahedral complexes with 3 bidentate ligands.
For example, the complex ion [Ni(NH2CH2CH2NH2)3]2+ can exist as two mirror images that cannot be superimposed:
Cis-trans isomers in complex ions
Cis-trans isomers are types of stereoisomers where identical groups can be positioned either adjacent (cis) or opposite (trans) to each other.
Cis-trans isomerism occurs in:
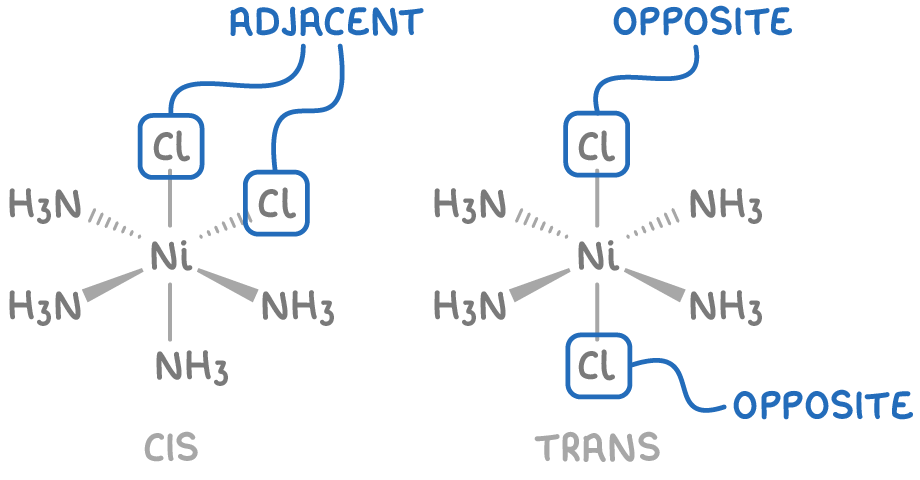
- Octahedral complexes with 4 monodentate ligands of one type and 2 monodentate ligands of another.
For example, in the complex ion Ni(NH3)4Cl2, the Cl- ligands are either adjacent to each other (cis-isomer) or on opposite corners of the octahedron (trans-isomer).
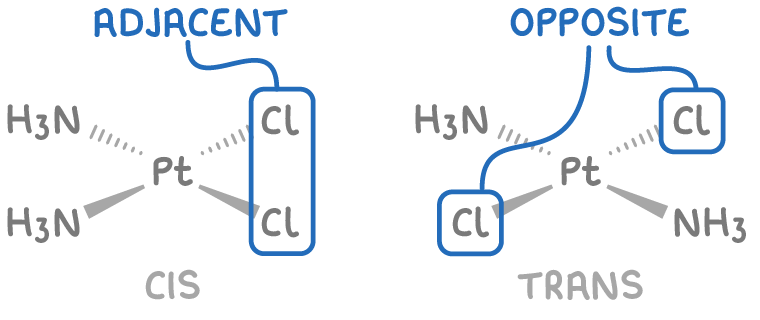
2. Square planar complexes with 2 pairs of monodentate ligands, for example, Pt(NH3)2Cl2.
For example, in the complex ion Pt(NH3)2Cl2, the Cl- ligands are either adjacent to each other (cis-isomer) or diagonally opposite to each other (trans-isomer).
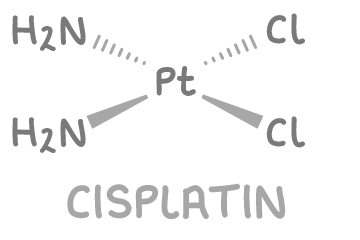
Cisplatin as an anti-cancer drug
Cisplatin, Pt(NH3)2Cl2, is a square planar platinum(II) complex with 2 chloride ligands and 2 ammonia ligands positioned next to each other (cis arrangement).
Cisplatin is used to treat certain cancers:
- The cis arrangement allows the chloride ligands to easily detach inside cancer cells.
- Cisplatin then binds to nitrogen atoms on the cancer cell's DNA via the platinum ion.
- This DNA binding prevents the cancer cell from reproducing through cell division.
- The cancer cell is unable to repair the DNA damage and eventually dies.
By interfering with the DNA replication process, cisplatin prevents cancer cells from multiplying.
However, cisplatin can also bind to DNA in healthy cells, especially rapidly dividing ones like hair follicles and bone marrow. This binding can prevent the cells from replicating properly.
This leads to several side effects, including:
- Hair loss - due to damage to hair follicle cells.
- Suppressed immune system - This is because of the impact on bone marrow cells, which are crucial for producing white blood cells.
- Kidney damage - This is because the kidneys work to filter and excrete the drug.
To mitigate these side effects, the dosage of cisplatin is kept low, and research is ongoing to develop methods that target the drug more precisely to cancer cells.
Despite its side effects, cisplatin remains a valuable tool in cancer treatment due to its effectiveness in stopping tumour growth. The treatment regimen is carefully planned to balance the benefits against the potential risks.