Nomenclature of Organic Compounds
This lesson covers:
- The meaning of nomenclature
- The key homologous series of organic compounds
- Naming aliphatic compounds
- Naming esters
- Naming aromatic compounds
What is nomenclature?
Nomenclature refers to the systematic method of naming chemical compounds in accordance with established rules. The IUPAC system is an internationally recognized set of guidelines for naming both organic and inorganic compounds. Understanding and applying these rules allows you to accurately name any organic molecule.
This lesson will guide you through the process of naming organic compounds using the following steps:
- Identifying the longest continuous carbon chain to determine the stem name.
- Recognising the key functional groups to determine suffix names.
- Numbering the carbon atoms based on the location of the functional group.
- Including numbers and prefixes for any side chains present.
- Applying multipliers like di-, tri-, and so on for multiple identical groups.
- Assembling the complete name by integrating all these elements.
Homologous series of organic compounds
Organic compounds can be categorised into homologous series based on similarities in their molecular structures.
Some of the main homologous series are listed below, along with the prefixes, suffixes and functional groups used to identify them:
| Homologous series | Functional group | Suffix | Example |
|---|---|---|---|
| Alkane | N/A | -ane | Ethane, C2H6 |
| Alkene | C=C | -ene | Ethene, C2H4 |
| Alcohol | O-H | -ol | Ethanol, C2H5OH |
| Aldehyde | C=O | -al | Ethanal, CH3CHO |
| Ketone | C=O | -one | Propanone, CH3COCH3 |
| Carboxylic acid | COOH | -oic acid | Ethanoic acid, CH3COOH |
| Amine | N-H | -amine | Ethylamine, CH3CH2NH2 |
| Nitrile | C≡N | -nitrile | Ethanenitrile, CH3CN |
| Amide | CON | -amide | Ethanamide, CH3CONH2 |
| Acyl chloride | COCl | -oyl chloride | Ethanoyl chloride, CH3COCl |
| Homologous series | Functional group | Prefix | Example |
|---|---|---|---|
| Branched alkanes | N/A | alkyl- | Methylpropane, (CH3)2CHCH3 |
| Cycloalkanes | N/A | cyclo- | Cyclohexane, C6H12 |
| Halogenoalkanes | C-X | fluoro-, chloro-, bromo-, iodo- | Chloroethane, CH3CH2Cl |
| Hydroxynitrile | C(OH)C≡N | hydroxy- | 2-hydroxypropanenitrile, CH3CH(OH)CN |
Naming aliphatic compounds
Step 1: Finding the base carbon chain
The first step in naming an organic molecule involves:
- Locating the longest continuous chain of carbon atoms that contains the primary functional group.
- The number of carbon atoms in this chain determines the stem name, as per the following table:
| Number of carbons | Stem name |
|---|---|
| 1 | Meth- |
| 2 | Eth- |
| 3 | Prop- |
| 4 | But- |
| 5 | Pent- |
| 6 | Hex- |
| 7 | Hept- |
| 8 | Oct- |
| 9 | Non- |
| 10 | Dec- |
For instance, in the illustrated compound below, the longest continuous carbon chain comprises 4 carbons, hence the stem name is but-.
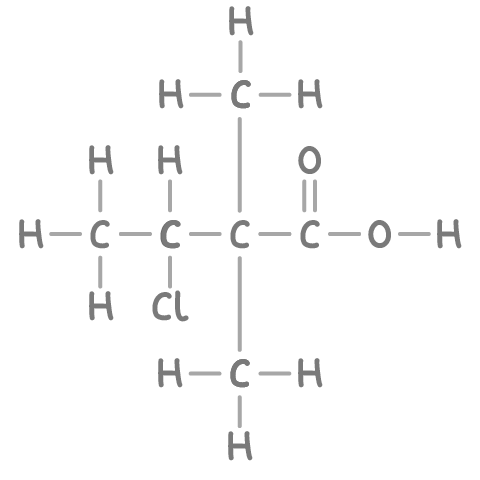
Step 2: Identifying the functional group
The next step is to identify the key functional group, which typically provides the suffix of the compound's name.
For example, the compound illustrated contains a carboxylic acid group (-COOH) on the first carbon, giving it the suffix -oic acid.
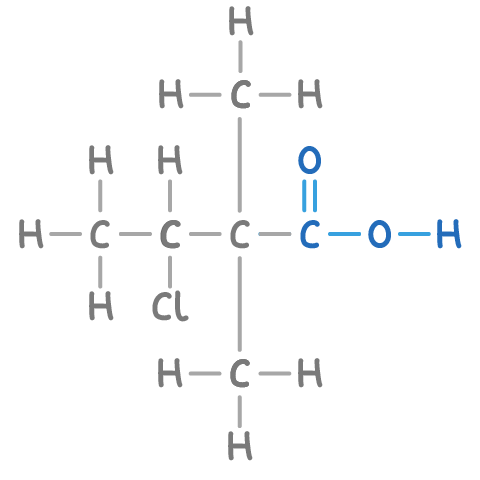
Step 3: Numbering the longest chain
The carbon atoms in the longest chain are numbered, with the primary functional group's carbon receiving the lowest possible number.
In our example, numbering the 4 carbon atoms with the -COOH group being assigned the lowest number results in a sequence from 1 to 4.
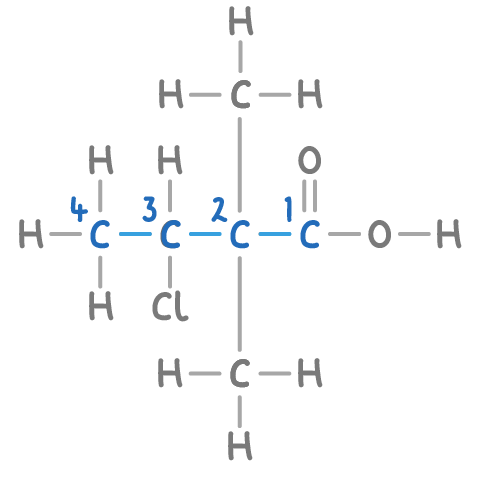
Step 4: Adding side chain prefixes
- Identify any side chains - these are smaller chains attached to the main carbon chain.
- Include these as prefixes in the name, indicating the carbon number to which they are attached.
- Arrange multiple side chains in alphabetical order.
In the compound shown, there is a chloro group on carbon 3 and methyl groups on carbon 2.
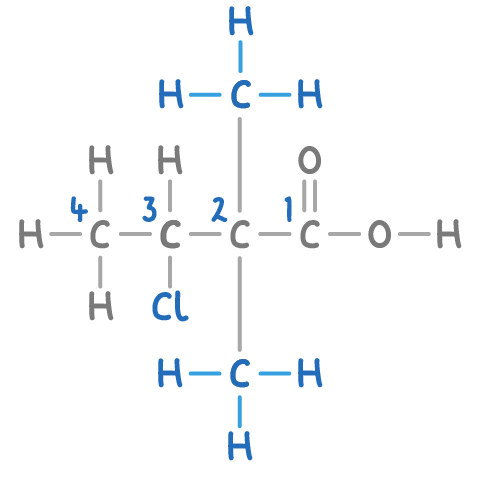
Step 5: Using multipliers
Use multipliers for multiple identical groups or side chains:
- di- for 2
- tri- for 3
- tetra- for 4
These multipliers do not affect the alphabetical order of the side chains.
In our example, the presence of two identical methyl groups is indicated by the prefix di-.
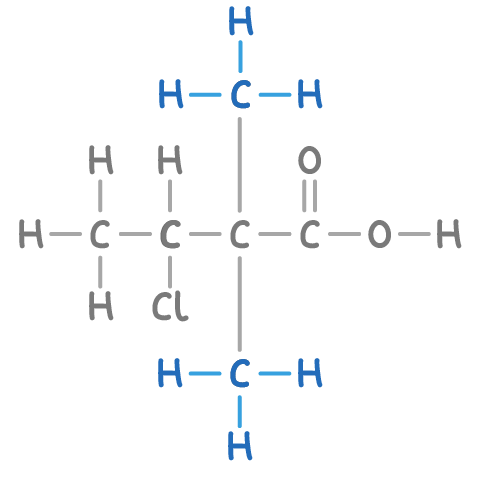
Step 6: Putting together the complete name
Combine all the information from the previous steps to name the compound systematically.
Thus, the compound's name is 3-chloro-2,2-dimethylbutanoic acid, comprising of:
- 3-chloro- = Chlorine atom on carbon 3
- 2,2-dimethyl- = Two methyl groups on carbon 2
- butan = Stem for 4 carbons
- oic acid = Carboxylic acid functional group
Worked example 1 - Naming an organic compound
Name the following compound.
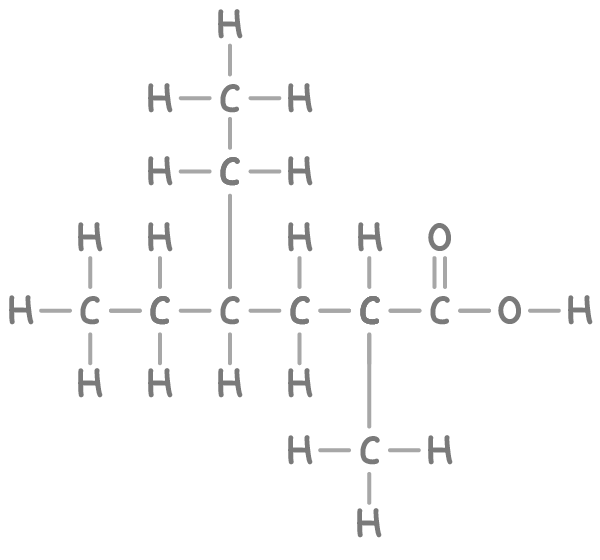
Step 1: Finding the base carbon chain
The longest continuous carbon chain in this compound consists of 6 carbons, hence the stem name is hex-.
Step 2: Identifying the functional group
The compound contains a carboxylic acid group (-COOH), providing the suffix -oic acid.
Step 3: Numbering the longest chain
Number the chain from the end closest to the carboxylic acid group for the lowest numbering. This gives a sequence from 1 to 6.
Step 4: Adding side chain prefixes
- The compound has an ethyl group on carbon 4.
- There is also a methyl group on carbon 2.
Step 5: Using multipliers
This compound does not have multiple identical groups, so no multipliers are used.
Step 6: Putting together the complete name
- 4-ethyl- = Ethyl group on carbon 4
- 2-methyl- = Methyl group on carbon 2
- hex = Stem for 6 carbons
- oic acid = Carboxylic acid functional group
Therefore, the name of the compound is 4-ethyl-2-methylhexanoic acid.
Worked example 2 - Naming an organic compound
Name the following compound.
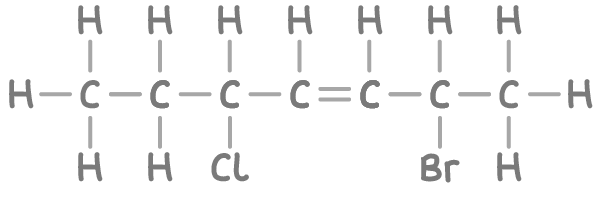
Step 1: Finding the base carbon chain
This compound's longest continuous carbon chain consists of 7 carbons, leading to the stem name hept-.
Step 2: Identifying the functional group
The compound has an alkene group (C=C) between carbons 3 and 4, resulting in the suffix -ene. It's important to indicate the position of the double bond.
Step 3: Numbering the longest chain
The chain is numbered to give the double bond and substituents (bromine and chlorine) the lowest possible numbers, resulting in a sequence from 1 to 7. The double bond is located between carbons 3 and 4.
Step 4: Adding side chain prefixes
- There is a bromine atom on carbon 2.
- A chlorine atom is attached to carbon 5.
Step 5: Using multipliers
There are no multiple identical groups, so multipliers are not needed.
Step 6: Putting together the complete name
- 2-bromo- = Bromine on carbon 2
- 5-chloro- = Chlorine on carbon 5
- hept = Stem for 7 carbons
- 3-ene = Alkene group starting at carbon 3
Therefore, the name of the compound is 2-bromo-5-chlorohept-3-ene.
Naming esters
Esters are formed from reacting an alcohol with a carboxylic acid.
Esters contain the functional group C-O-C=O.
Rules for naming esters:
- Identify the alkyl group from the alcohol - this gives the first part of the ester name.
- Identify the acid group and replace the '-oic acid' ending with '-oate' - this gives the second part of the name.
- Put the two parts together to give the complete ester name.
For example, the ester below is named propyl ethanoate:
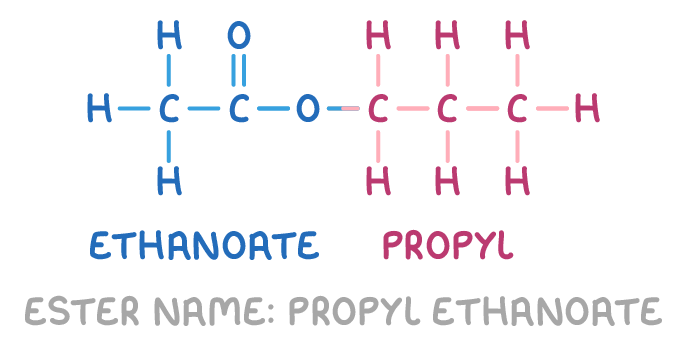
This is because:
- It contains a 3-carbon alkyl chain from the alcohol (indicated in red). This is recognised as a propyl group.
- It contains a 2-carbon chain from the acid (indicated in blue) with the ester bond -COO- in the middle. Converting the acid name ethanoic acid to an -oate ending gives ethanoate.
- Putting the two parts together, the alcohol-derived propyl group at the front and the acid-derived ethanoate group at the end, gives the complete name propyl ethanoate.
With branched acids or alcohols:
- Number the α-carbon atom next to the ester functional group as carbon 1.
- Name other substituents based on their position
For example, the ester below is named ethyl 3-methylbutanoate:
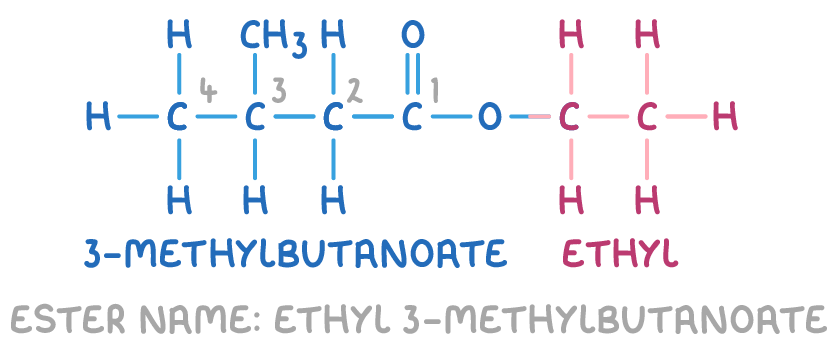
Naming aromatic compounds
Aromatic compounds containing benzene rings are called arenes.
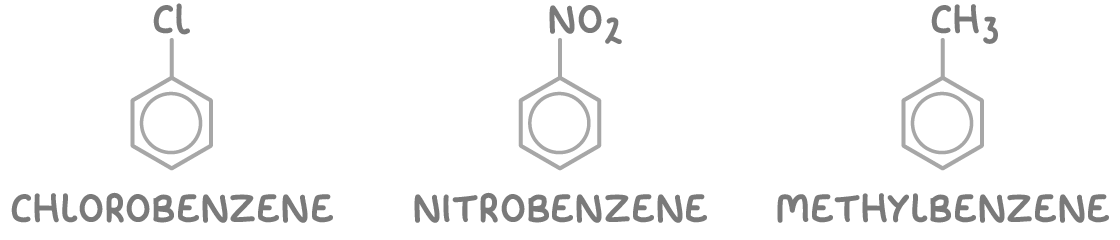
Rules for naming arenes:
1. Some arenes are named as substituted benzene derivatives using the suffix -benzene.
These include:
- Halogenoarenes (e.g. chlorobenzene)
- Nitroarenes (e.g. nitrobenzene)
- Alkylarenes (e.g. methylbenzene)
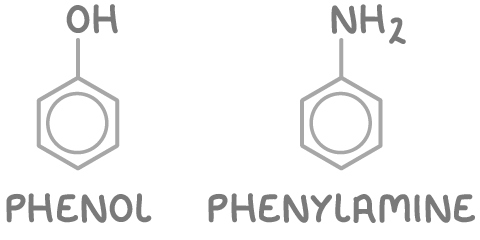
2. Others arenes are named as phenyl (C6H5) derivatives using prefix phenyl-.
These include:
- Phenol
- Phenylamine
3. Arenes with multiple substituents are named by numbering the carbon atoms in the benzene ring such that the substituents receive the lowest possible numbers. The numbering begins at the carbon atom with the functional group that determines the suffix of the compound's name.
For example, the arene below is named 2,4-dibromophenylamine:
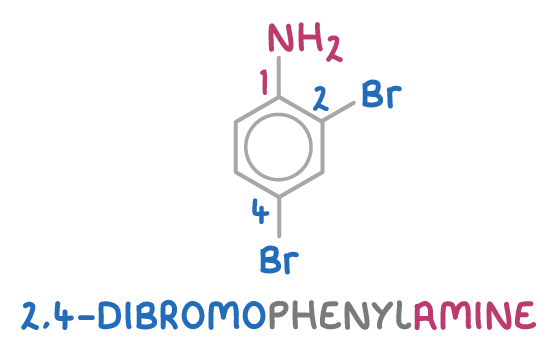
This is because:
- It contains an -NH2 functional group, which means the compound is an amine. Therefore, the suffix should be -phenylamine based on the presence of the amino group.
- Numbering from the amino group gives the bromine substituents positions 2 and 4. Therefore, the full name of this compound is 2,4-dibromophenylamine.