Determination of Relative Mass
This lesson covers:
- Interpreting mass spectra
- Calculating relative atomic mass from mass spectra
Interpreting mass spectra
A mass spectrum plots the relative abundance of ions against their mass-to-charge ratio (m/z).
- The x-axis displays the m/z values. The m/z of each peak equals the relative mass of the ion.
- The y-axis indicates the relative abundance of each ion, either in arbitrary units or as a percentage.
For elemental samples, each peak represents a different isotope.
- The relative height of each peak shows how abundant the isotope is.
- Elements with only one stable isotope will show a single peak.
Below is the mass spectrum of an element with two stable isotopes.
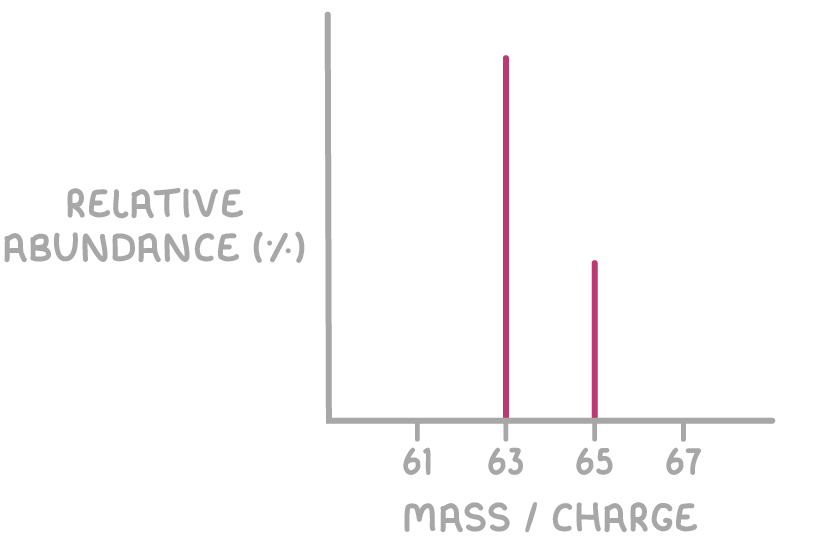
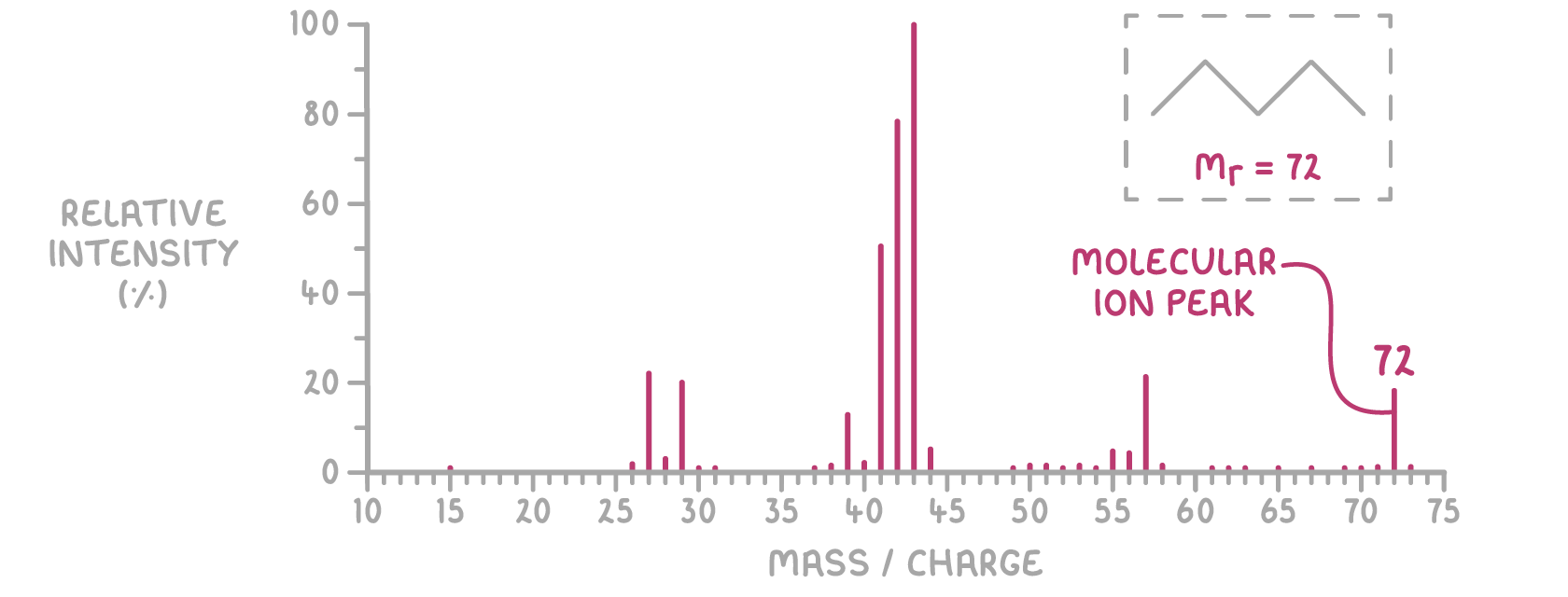
For molecular samples, the peak at the highest m/z value is the molecular ion (M+).
- The m/z of the molecular ion peak matches the molecule's relative molecular mass (Mr).
- Peaks at lower m/z values come from fragments of the molecular ion.
Below is the mass spectrum for a compound with a molecular mass of 72.
Calculating relative atomic mass from mass spectra
To find an element's relative atomic mass (Ar) from its mass spectrum, follow these steps:
- Multiply the relative isotopic mass by its relative abundance for each isotope.
- Add these products together.
- Divide the total by the sum of the relative abundances (100 if using percentages).
Worked example 1 - Calculating the relative atomic mass of copper from its mass spectrum
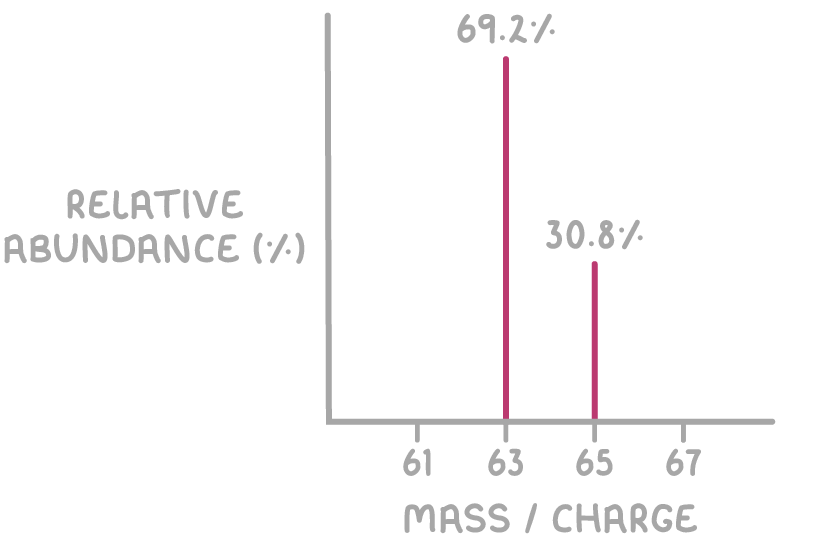
Calculate the relative atomic mass (Ar) of copper using the mass spectrum shown below:
Step 1: Multiply the relative isotopic mass by its relative abundance for each isotope
For 63Cu: 63 x 69.2 = 4,359.6
For 65Cu: 65 x 30.8 = 2,002.0
Step 2: Sum these values
Sum = 4,359.6 + 2,002.0 = 6,361.6
Step 3: Divide by the total relative abundance
Total relative abundance = 69.2 + 30.8 = 100.0
Ar =1006,361.6=63.616
Therefore, the calculated relative atomic mass (Ar) of copper from its mass spectrum is 63.6.