Giant Covalent Structures
This lesson covers:
- What giant covalent structures are
- Diamond - bonding, structure, properties
- Graphite - bonding, structure, properties
- Graphene - bonding, structure, properties
Giant covalent structures
Some elements can form extensive interconnecting networks of covalently bonded atoms known as giant covalent structures.
- These structures involve huge lattices extending in three dimensions.
- In carbon, the small atomic size and ability to form 4 covalent bonds per atom allow the formation of giant covalent structures.
- The different structural forms of an element in the same state are called allotropes.
The 3 allotropes of carbon with giant lattice structures that you need to know about are diamond, graphite, and graphene.
Diamond allotrope
Bonding and structure:
Each carbon atom forms 4 very strong covalent bonds with others in a tetrahedral arrangement.
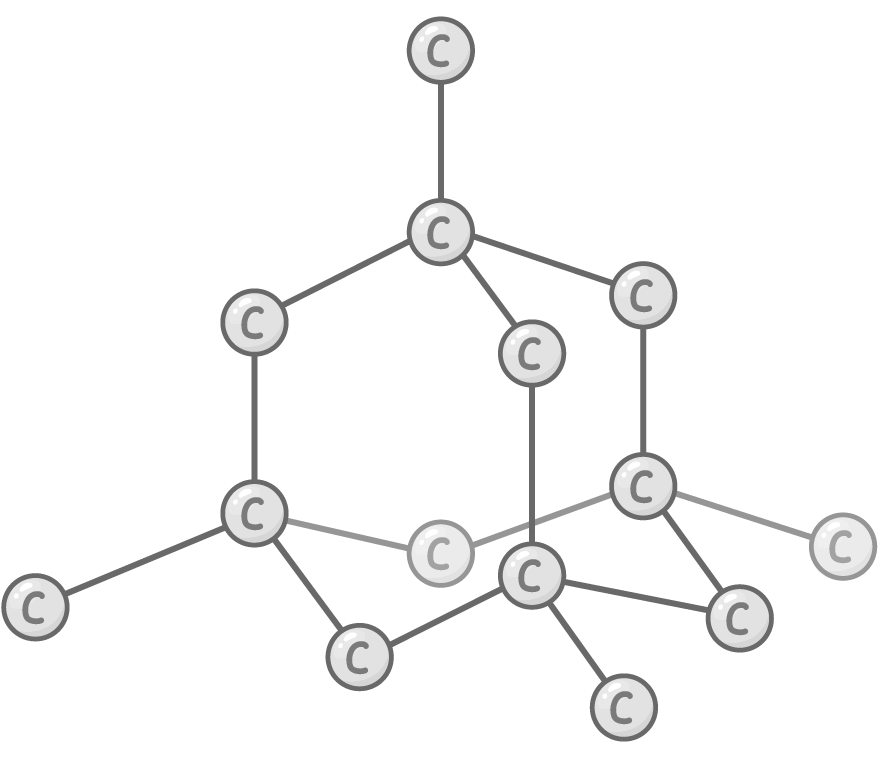
Properties:
- Extremely hard - Extensive network of strong covalent bonds not easily broken
- Very high melting point - Huge amount of energy needed to break enough bonds to melt diamond
- Good thermal conductor - Strong interatomic bonds transmit heat through vibrations
- Electrical insulator - All outer electrons tied up in localised bonds so no free electrons to carry charge
- Insoluble - Covalent bonds too strong to be broken by solvation
Silicon, another group 4 element, also forms a similar giant covalent structure where each silicon atom forms 4 strong covalent bonds in a tetrahedral arrangement. Like diamond, silicon has a high melting point, is very hard, and is insoluble due to its network of strong covalent bonds between atoms.
Graphite allotrope
Bonding and structure:
- Each carbon atom forms 3 strong covalent bonds in a planar hexagonal pattern, with each carbon contributing 1 delocalised electron.
- Multiple stacked layers of hexagonal carbon arrays with weak intermolecular forces between layers.
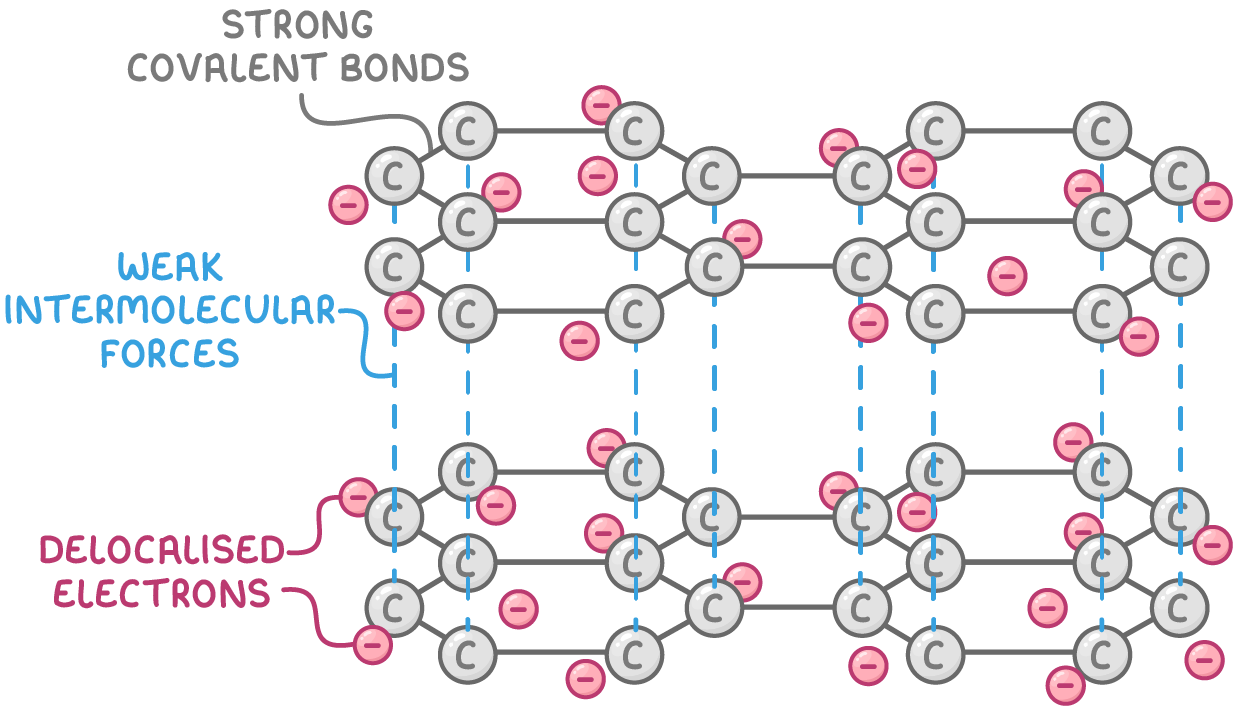
Properties:
- Soft and slippery - Weak intermolecular forces let sheets slide over each other
- Conducts electricity along layers - Delocalised electrons move through the 2D lattice carrying electrical charge
- Lower density than diamond - Weak intermolecular forces lead to increased separation between layers
- High sublimation temperature but lower melting point than diamond - Covalent bonds within each layer are very strong but the weaker intermolecular forces between layers means graphite melts at a lower temperature
Graphene allotrope
Bonding and structure:
Graphene consists of a single layer of carbon atoms interconnected through strong planar covalent bonds in a hexagonal pattern, with each carbon contributing 1 delocalised electron. This essentially forms a one-atom thick slice of graphite.
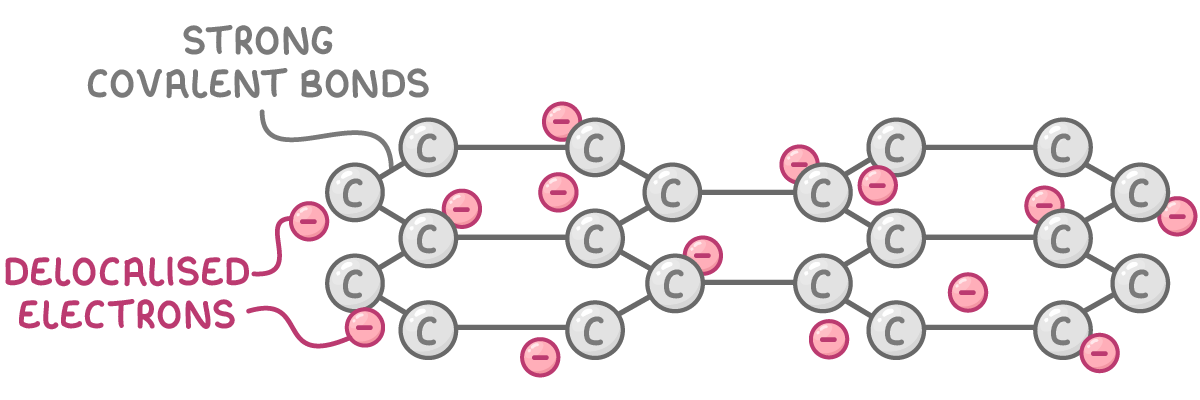
Properties:
- Excellent electrical and thermal conductivity - Delocalised electrons move through the 2D lattice transporting heat and charge
- Very strong - Extensive network of covalent bonds not easily broken
- Transparent and extremely lightweight - A single layer of atoms light and thin enough to transmit visible light
Summary
The table below summarises the bonding, structure and properties of diamond, graphite and graphene.
| Diamond | Graphite | Graphene | |
|---|---|---|---|
| Bonding | 4 strong 3D covalent bonds per carbon atom in tetrahedral arrangement | 3 strong planar covalent bonds + 1 delocalised electron per carbon. Weaker interlayer forces | 3 strong planar covalent bonds + 1 delocalised electron per carbon |
| Structure | 3D network of tetrahedrally bonded carbon atoms | Stacked 2D hexagonal carbon sheets | Single layer of hexagonally arranged carbon atoms |
| Properties | Extremely hard / Very high melting point / Good thermal conductor / Electrical insulator / Insoluble | Softer and layers slide / Conducts electricity in layers / Lower density than diamond / High sublimation temperature | Excellent electrical and thermal conductivity / Very strong / Transparent and lightweight |