Periodic Trends in Bonding and Structure
This lesson covers:
- How bond strength affects melting and boiling points across a period
- Trends in atomic radius across a period
Melting and boiling points reflect structure and bonding across periods
The type of bonds and strength of bonds between atoms affects the melting and boiling points of elements across periods 2 and 3.
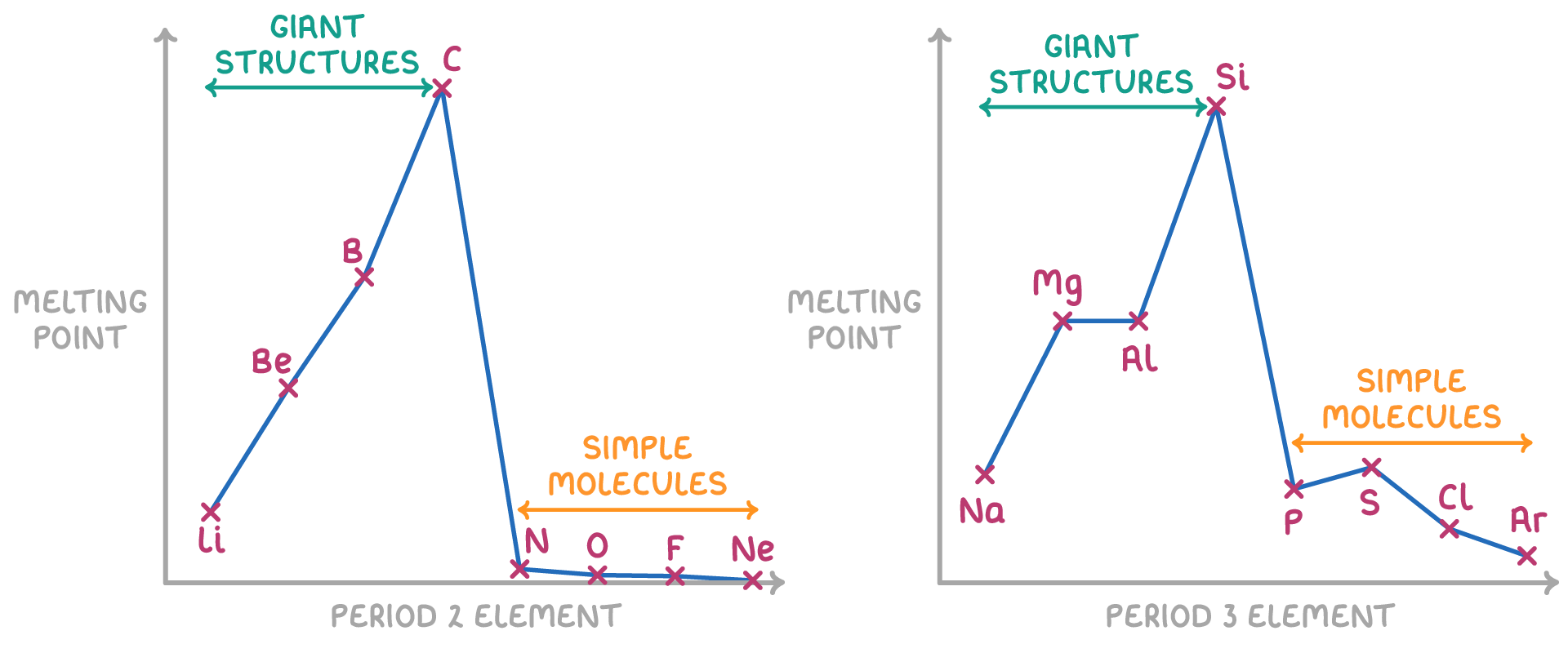
- For the metals lithium (Li), beryllium (Be), sodium (Na), magnesium (Mg) and aluminium (Al), the melting and boiling points increase across the period due the increasing strength of the metallic bonding.
The strength of metallic bonding increases across the period because:
- The ions have a larger positive charge (1+, 2+, 3+).
- The ions have a smaller ionic radius.
- There will be more delocalised electrons (e.g. Na only has 1 delocalised electron per ion, but Al has 3 per ion).
- These three factors all result in a stronger electrostatic attraction between metal cations and delocalised electrons which requires increasing amounts of energy to overcome.
- The elements carbon (C) and silicon (Si) have giant covalent lattice structures. Here, each atom is covalently bonded to 4 neighboring atoms in a tetrahedral arrangement. This forms very strong bonds linking all the atoms together in sheets (e.g. graphite) or 3D lattices (e.g. diamond). A huge amount of energy is required to break these covalent bonds, resulting in extremely high melting and boiling points.
- Nitrogen (N2), oxygen (O2), fluorine (F2), phosphorus (P4), sulfur (S8), and chlorine (Cl2) have simple molecular structures, with only weak induced dipole-dipole forces existing between the molecules. These intermolecular forces are easily overcome, requiring little energy, and so they result in low melting and boiling points.
- The noble gases neon (Ne) and argon (Ar) have the lowest melting and boiling points. Their atoms do not form any bonds and only very weak induced dipole-dipole forces attract them. Minimal energy is needed to overcome these negligible forces between atoms.
Atomic radius decreases across a period
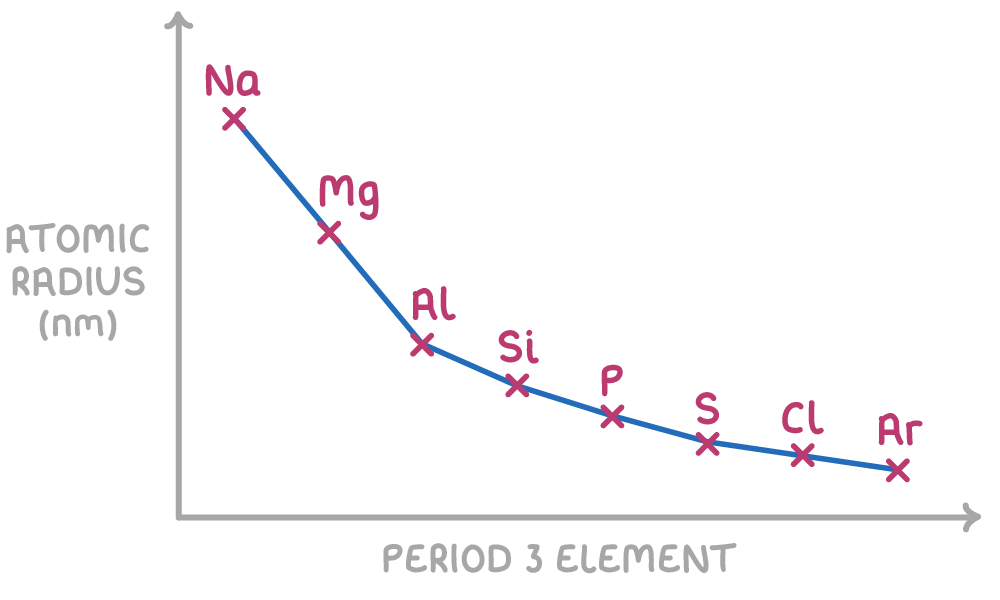
Atomic radius decreases across a period because:
- As protons are added across a period, the nuclear charge increases. This results in a stronger electrostatic attraction between the nucleus and the outer electrons, drawing the outer electrons closer to the nucleus.
- The electrons added across a period go into the outer energy level, so provide little additional shielding for inner electrons.
- With increasing nuclear charge and minimal change in shielding, the stronger electrostatic attraction causes the atomic radius to decrease across the period.