Introducing Benzene
This lesson covers:
- The structure and bonding in benzene
- The delocalised model of benzene
- Evidence supporting the delocalised model
- Naming aromatic compounds
- Differences in reactivity between benzene and alkenes
Benzene has a planar ring structure
Arenes are aromatic hydrocarbons that contain a benzene ring. Benzene is the simplest arene and has a planar ring structure. Benzene, with the molecular formula C6H6, is composed of a hexagonal ring that includes six carbon atoms.
Each of these carbon atoms is bonded to the following:
- One hydrogen atom.
- Two other carbon atoms adjacent to it within the ring.
To depict benzene's structure, there are two primary models:
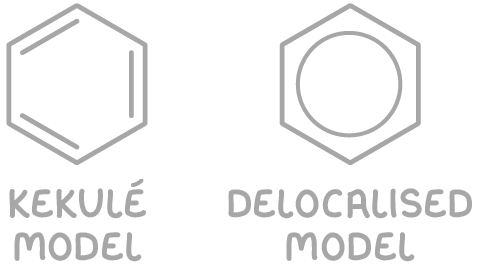
- The Kekulé model - This represents the structure with alternating single and double bonds.
- The delocalised model - This shows a ring of electrons that are delocalised.
How the delocalised model is formed
The delocalised model arises from overlap of the p-orbitals on the six carbon atoms in benzene:
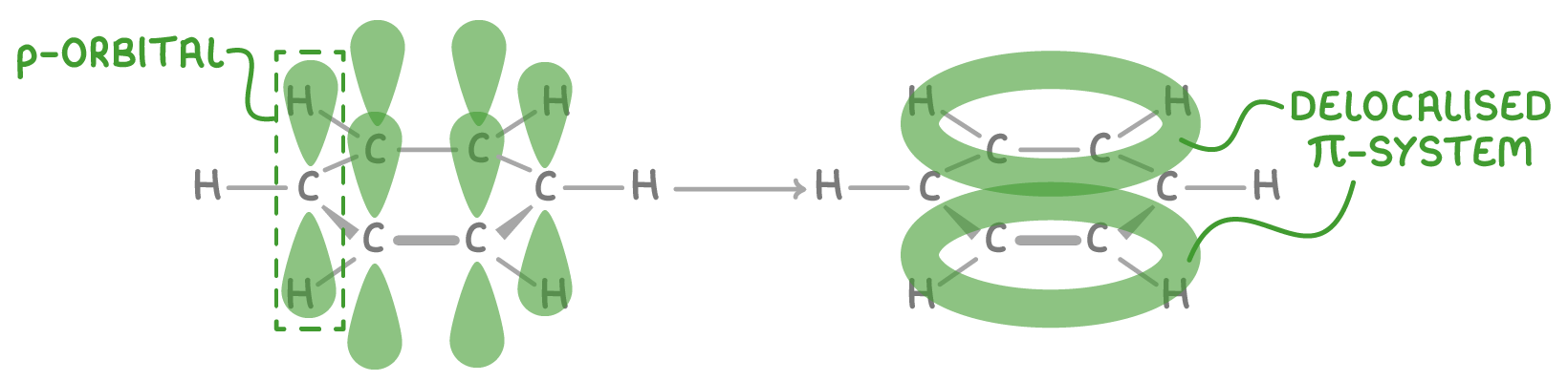
- Each carbon contributes one electron from its 2p orbital to a π-bonding system.
- The p-orbitals overlap side-by-side around the ring, forming a delocalised system of 6 π-electrons.
- This creates an electron density above and below the plane of carbon atoms.
- The electrons are not fixed between specific atom pairs, but rather delocalised over the whole ring.
This delocalisation leads to equal C-C bond lengths between the carbon atoms and enhanced stability of the aromatic ring.
Evidence supports the delocalised model
The delocalised model of benzene is strongly supported by scientific evidence.
- Equivalent carbon-carbon bond lengths
- X-ray diffraction techniques have revealed that all carbon-carbon (C-C) bonds in benzene measure 140 pm in length.
- This measurement sits between the length of a typical C-C single bond (134 pm) and that of a C=C double bond (154 pm).
- Such findings contradict the Kekulé model, which would suggest alternating lengths for single and double bonds.
- Enthalpy of hydrogenation
- Hydrogenation of cyclohexene, which has one C=C bond, results in a change in enthalpy (ΔH) of -120 kJ mol-1.
- If benzene had three double bonds, as suggested by the Kekulé model, it would have a ΔH of -360 kJ mol-1 following the same logic.
- However, the actual ΔH for benzene's hydrogenation is only -208 kJ mol-1.
- This indicates that breaking the bonds in benzene requires more energy, suggesting a stability greater than what the Kekulé model predicts.
- Resistance to electrophilic addition reactions
- Unlike alkenes, which readily undergo electrophilic addition reactions (e.g., decolourising bromine water at room temperature), benzene is resistant to such reactions.
- This resistance is due to the delocalised π-electron system, which stabilises the benzene ring and makes it less reactive towards electrophiles.
- The Kekulé model, with its alternating double bonds, would predict benzene to be more reactive, similar to alkenes.
This remarkable stability seen in benzene is attributed to the delocalisation of electrons above and below the hexagonal ring.
Naming aromatic compounds
Compounds that include a benzene ring are referred to as arenes or aromatic compounds.
There are two main systems used for naming these compounds:
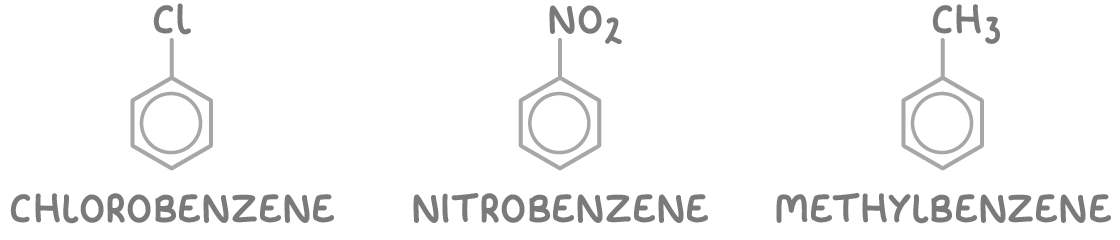
1. Substituted benzene - Here, the names of the substituents precede the word "benzene". Examples include chlorobenzene, nitrobenzene, and methylbenzene.
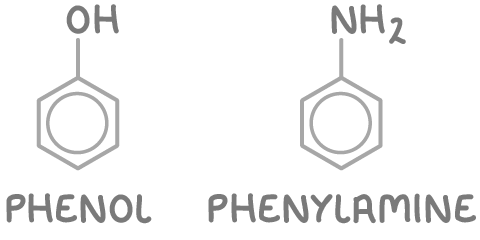
2. Phenyl derivatives - These compounds are named as derivatives of the phenyl group (C6H5-). Examples include phenol and phenylamine.
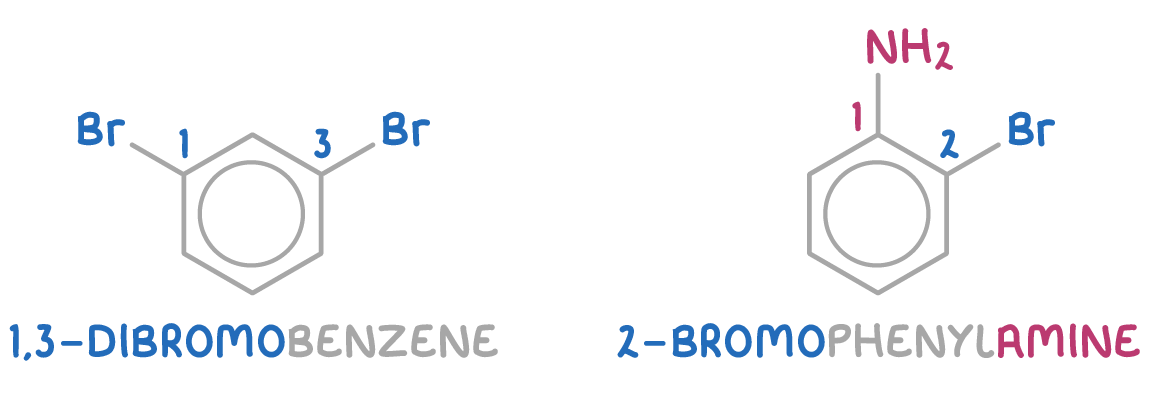
When multiple substituents are present, the positions on the ring are numbered to clarify their locations:
- The numbering begins from the substituent that gives the molecule its suffix (for example, -OH in phenol).
- If all substituents are identical, numbering starts from any position and proceeds to give the lowest possible numbers.
Examples include 1,3-dibromobenzene and 2-bromophenylamine:
Differences in benzene and alkene reactivity
Benzene and alkenes exhibit significant differences in their chemical reactivities.
Alkenes readily undergo addition reactions
- Alkenes are known for their readiness to undergo addition reactions with electrophiles, such as bromine, by breaking the π-bond in the C=C double bond.
- For instance, ethene reacts with bromine at room temperature to produce 1,2-dibromoethane:
C2H4 + Br2 ➔ CH2BrCH2Br
Benzene prefers substitution over addition
- Addition reactions in benzene are difficult due to the stability provided by its delocalised π-electron system.
- Instead, benzene is more inclined to participate in substitution reactions, which preserve the aromatic ring's integrity.
- For instance, benzene reacts with bromine when heated in the presence of a catalyst to form bromobenzene and hydrogen bromide:
C6H6 + Br2 ➔ C6H5Br + HBr
Delocalisation explains reactivity differences
- In benzene, the delocalised π-system across the ring has insufficient electron density to polarise the Br-Br bond, making addition reactions difficult. Heat and a catalyst are required to initiate the substitution reaction.
- In ethene, the localised π-system around the C=C double bond has sufficient electron density to polarise the Br-Br bond, allowing addition reactions to occur readily at room temperature without the need for a catalyst.