Electrophilic Substitution Reactions in Benzene
This lesson covers:
- The mechanism of electrophilic substitution in arenes
- How halogen carriers produce reactive electrophiles
- Halogenation of benzene
- Nitration of benzene
- Friedel-Crafts alkylation reactions to form C-C bonds
- Friedel-Crafts acylation reactions to form C-C bonds
Benzene undergoes electrophilic substitution
Electrophilic substitution reactions of benzene involve a hydrogen atom being replaced by an electrophile.
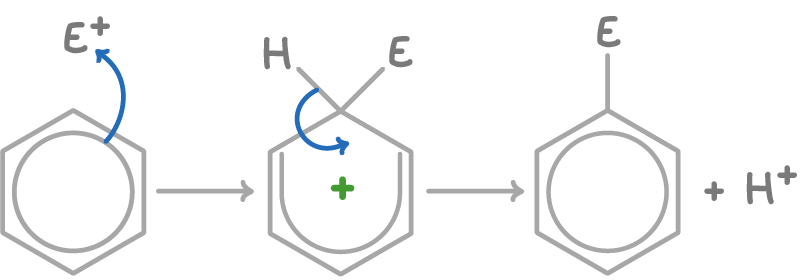
The mechanism unfolds in two stages:
- Addition of the electrophile (E+) to the benzene ring - This breaks the aromaticity and forms a positively charged intermediate.
- Loss of H+ - This step reforms the aromatic benzene ring with the electrophile substituted in place of hydrogen.
Halogen carriers help make good electrophiles
An electrophile has to have a pretty strong positive charge to be able to attack the stable benzene ring. Most compounds just aren't polarised enough — but some can be made into stronger electrophiles using a catalyst called a halogen carrier.
A halogen carrier accepts a lone pair of electrons from a halogen atom on an electrophile. As the lone pair of electrons is pulled away, the polarisation in the molecule increases and sometimes a carbocation forms. This makes the electrophile stronger.
Halogen carriers include aluminium halides, iron halides and iron.
For example, AlCl3 can accept a lone pair from chlorine in Cl2, generating the more electrophilic Cl+ species:
AlCl3 + Cl2 ➔ Cl+ + AlCl4-
Halogenation of benzene
Halogenation refers to the electrophilic substitution of a halogen atom onto a benzene ring in place of hydrogen. It can be carried out by reacting benzene with halogen molecules in the presence of halogen carrier catalysts like aluminium chloride (AlCl3) or iron chloride (FeCl3).
The chlorination of benzene using Cl2 and an AlCl3 catalyst occurs as:
- Chlorine reacts with the aluminium chloride catalyst to form the electrophilic chloronium (Cl+) ion:
Cl2 + AlCl3 ➔ Cl+ + AlCl4-
- Benzene undergoes electrophilic substitution with the electrophile Cl+, displacing a proton and forming chlorobenzene:
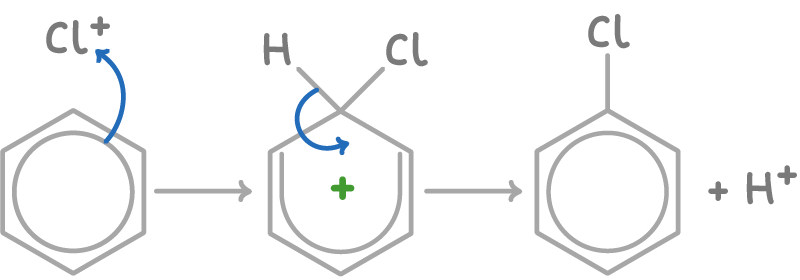
3. The displaced proton reacts with AlCl4- to regenerate the AlCl3 catalyst:
H+ + AlCl4- ➔ AlCl3 + HCl
A similar process occurs for the bromination of benzene using bromine and catalysts like AlBr3 or FeBr3.
Nitration of benzene
Nitration refers to the electrophilic substitution of a nitro group (-NO2) onto a benzene ring in place of hydrogen. It can be carried out by reacting benzene with concentrated nitric acid in the presence of concentrated sulfuric acid catalyst a temperature of 60°C or less.
The nitration of benzene using HNO3 and H2SO4 occurs as:
- Nitric acid reacts with the sulfuric acid catalyst to generate the electrophilic nitronium (NO2+) ion:
HNO3 + H2SO4 ➔ NO2+ + HSO4- + H2O
- Benzene undergoes electrophilic substitution with the electrophile NO2+, displacing a proton and forming nitrobenzene:
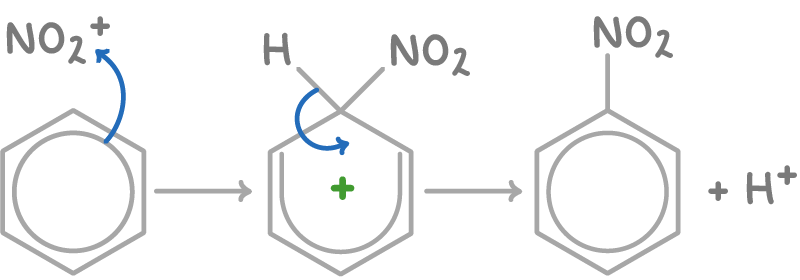
3. The displaced proton reacts with HSO4- to regenerate the H2SO4 catalyst:
H+ + HSO4- ➔ H2SO4
Controlling nitration
Cooling the nitration reaction to less than 60°C ensures that only mononitration occurs, producing nitrobenzene as the major product.
Friedel-Crafts reactions form C-C bonds
Friedel-Crafts reactions are really useful for forming C–C bonds in organic synthesis. They are carried out by refluxing benzene with a halogen carrier and either a halogenoalkane or an acyl chloride.
There are two types:
- Friedel-Crafts alkylation puts any alkyl group onto a benzene ring using a haloalkane and a halogen carrier.
- Friedel-Crafts acylation substitutes an acyl group for an H atom on benzene using an acyl chloride and halogen carrier. This produces phenylketones or benzaldehyde.
Friedel-Crafts alkyation mechanism
The alkylation of benzene using chloromethane and AlCl3 proceeds via:
- Formation of the electrophilic methyl carbocation (CH3+) intermediate:
CH3Cl + AlCl3 ➔ CH3+ + AlCl4-
- Electrophilic substitution occurs on benzene with CH3+, displacing H+ and forming methylbenzene:
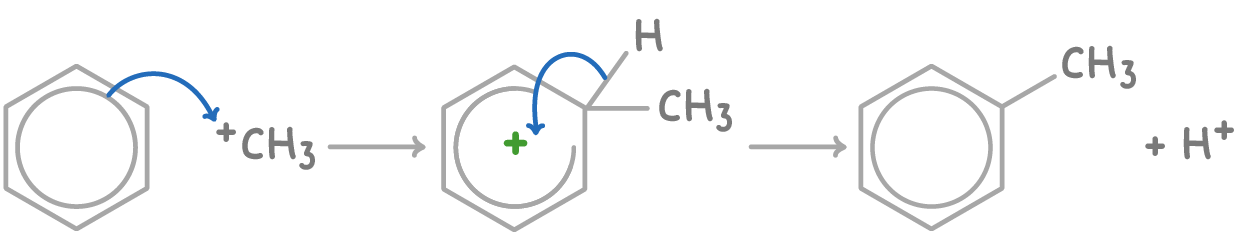
3. The proton reacts with AlCl4-, regenerating the AlCl3 catalyst:
H+ + AlCl4- ➔ AlCl3 + HCl
Friedel-Crafts acylation mechanism
The acylation of benzene using ethanoyl chloride and AlCl3 proceeds via:
- Formation of the electrophilic acetyl cation (CH3CO+) intermediate:
CH3COCl + AlCl3 ➔ CH3CO+ + AlCl4-
- Electrophilic substitution occurs on benzene with CH3CO+, displacing H+ and forming phenylethanone:
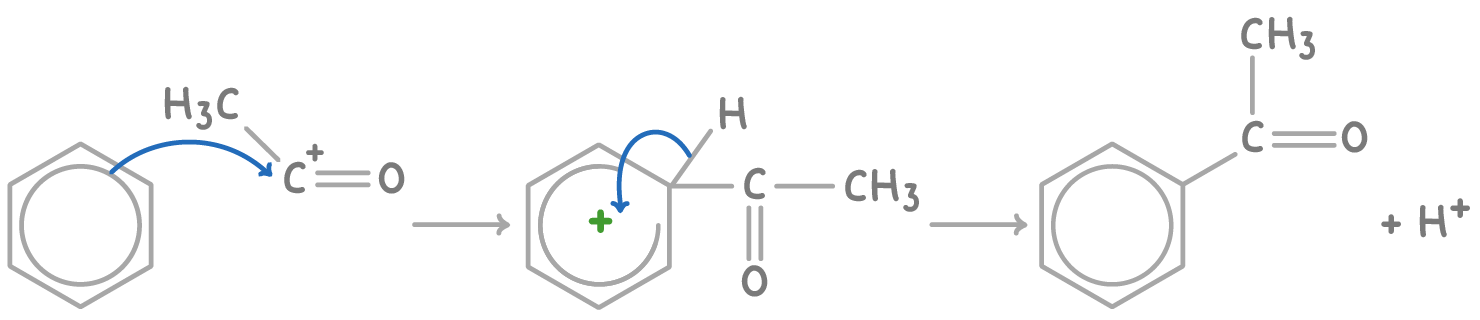
3. The proton reacts with AlCl4-, regenerating the AlCl3 catalyst:
H+ + AlCl4- ➔ AlCl3 + HCl