The Periodic Table
This lesson covers:
- How early chemists grouped elements by properties and atomic mass
- The contributions of Mendeleev and Moseley to the development of the periodic table
- The arrangement of the modern periodic table into blocks, periods and groups
- How to use the periodic table to determine the electron configuration of elements
Early attempts at organising elements
In the early 1800s, chemists could only categorise elements in two ways:
- By physical and chemical properties
- By relative atomic mass
- Johann Döbereiner identified triads of elements with similar properties and relative atomic masses in between the others.
- John Newlands organised elements by increasing atomic mass and noticed a pattern of similar elements recurring every 8 elements. He called this the law of octaves.
Döbereiner’s triads:
| Triad 1 | Triad 2 | Triad 3 |
|---|---|---|
| Li | Ca | Cl |
| Na | Sr | Br |
| K | Ba | I |
Newland’s octaves:
| Octave 1 | Li | Be | B | C | N | O | F |
|---|---|---|---|---|---|---|---|
| Octave 2 | Na | Mg | Al | Si | P | S | Cl |
However, these early attempts had limitations and did not reliably categorise all known elements.
Mendeleev's periodic table
In 1869, Dmitri Mendeleev created a better periodic table by:
- Primarily organising elements by increasing atomic mass
- Swapping the order of some elements to keep those with similar properties in the same group
- Leaving gaps where elements should fit based on trends in properties
- Predicting new elements to fit in these gaps
When the predicted elements were discovered and found to fit his table, it confirmed that Mendeleev had arranged elements logically based on their properties. His decision to prioritise properties over strict atomic mass order was later validated by the discovery of isotopes, which explained why some elements had different atomic masses but similar chemical behavior.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | |
|---|---|---|---|---|---|---|---|
| Period 1 | H | ||||||
| Period 2 | Li | Be | B | C | N | O | F |
| Period 3 | Na | Mg | Al | Si | P | S | Cl |
| Period 4 | K, Cu | Ca, Zn | *, * | Ti, * | V, As | Cr, Se | Mn, Br |
| Period 5 | Rb, Ag | Sr, Cd | Y, In | Zr, Sn | Nb, Sb | Mo, Te | *, I |
The positions marked * in the periodic table represent gaps that Mendeleev left for elements that had not yet been discovered at the time he created his periodic table.
Moseley improved the table based on atomic number
In 1914, Henry Moseley reorganised the periodic table by:
- Arranging elements in order of increasing atomic (proton) number.
- This properly sequenced the elements.
The modern periodic table is based on Moseley's version organised by atomic number.
Periodic table structure
The periodic table is organised into periods (rows) and groups (columns):
- Periods: Elements in the same period have the same number of electron shells. As you move down a period, the number of electron shells increases.
- Groups: Elements in the same group have similar electron configurations in their outer shell. As a result, they exhibit similar physical and chemical properties.
The periodic table can be further divided into three blocks based on the type of subshell being filled in the outermost electron shell:
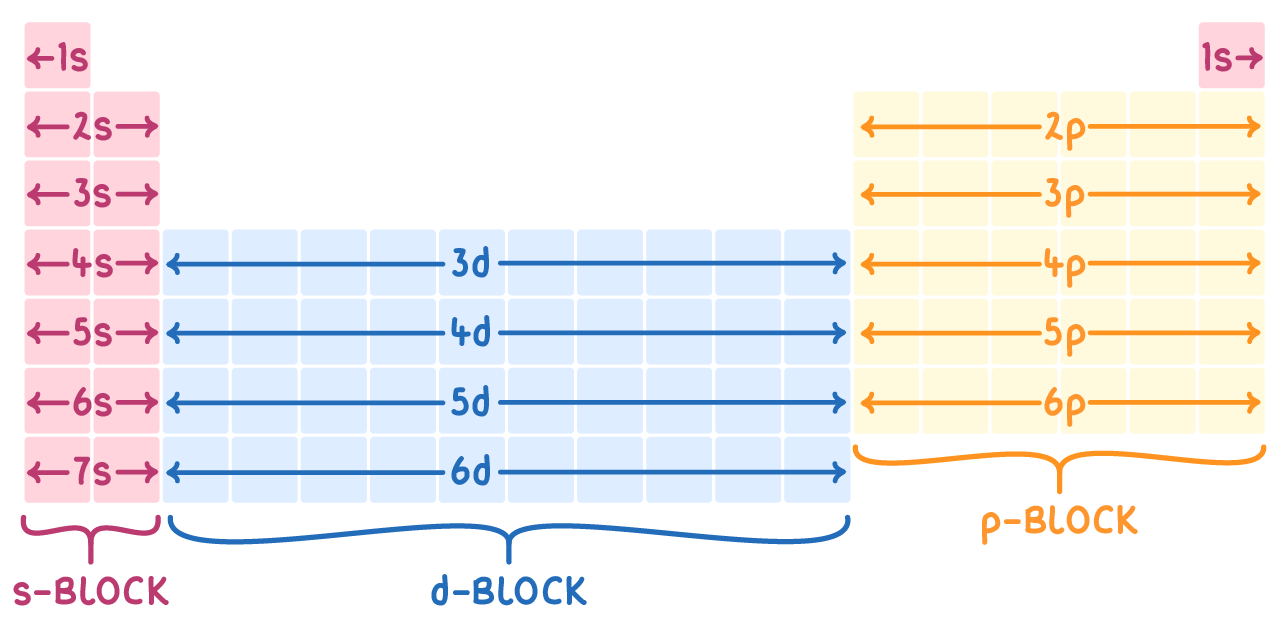
- s-block: Elements in the s-block have their outermost electrons in an s orbital.
For example, the electronic configuration of lithium (Li) is 1s2 2s1.
- p-block: Elements in the p-block have their outermost electrons in an p orbital.
For example, the electronic configuration of oxygen (O) is 1s2 2s2 2p4.
- d-block: Elements in the d-block have their outermost electron in a d orbital.
For example, the electronic configuration of iron (Fe) is 1s2 2s2 2p6 3s2 3p6 4s2 3d6.
Determining electron configurations
You can use the table to deduce the electron configuration of elements:
- Identify the period - This shows how many electron shells
- Note which block it falls in - This shows which subshell is being filled in the outer electron shell.
- Write out the complete configuration by building up from the 1s orbital
Worked example 1 - Determining the electron configuration of vanadium
Write the electronic configuration of an atom of vanadium, V.
Step 1: Identify which period vanadium belongs to
Vanadium is located in period 4, so it has 4 electron shells.
Step 2: Identify which block vanadium belongs to
Vanadium is part of the d-block, so its outer shell includes a partially filled d subshell.
Step 3: Write the complete electronic configuration
The complete configuration is:
Period 1: 1s2
Period 2: 2s2 2p6
Period 3: 3s2 3p6
Period 4: 4s2 3d3
Therefore, the electron configuration for vanadium is 1s2 2s2 2p6 3s2 3p6 4s2 3d3.
The abbreviated configuration is [Ar] 4s2 3d3.