Enthalpy Changes
This lesson covers:
- What enthalpy change is
- The difference between exothermic and endothermic reactions
- Enthalpy profile diagrams
- Standard conditions
- Types of enthalpy change
Introduction to enthalpy change
When chemical bonds break or form during a reaction, energy is often released or absorbed as heat. This heat energy change, occuring at constant pressure, is known as the enthalpy change (ΔH).
Enthalpy change is measured in kilojoules per mole (kJ mol-1).
Exothermic versus endothermic reactions
Exothermic reactions:
- Release heat energy to the surroundings
- Have a negative enthalpy change (ΔH is negative)
- Temperature of the surroundings increases
For example, the combustion of methane is exothermic:
CH4 + 2O2 ➔ CO2 + 2H2O, ΔH⦵r = -890 kJ mol-1
Endothermic reactions:
- Absorb heat energy from the surroundings
- Have a positive enthalpy change (ΔH is positive)
- Temperature of the surroundings decreases
For example, the thermal decomposition of calcium carbonate is endothermic:
CaCO3 ➔ CaO + CO2, ΔH⦵r = +178 kJ mol-1
Enthalpy profile diagrams
Enthalpy profile diagrams show how the energy levels of reactants and products change during a reaction.
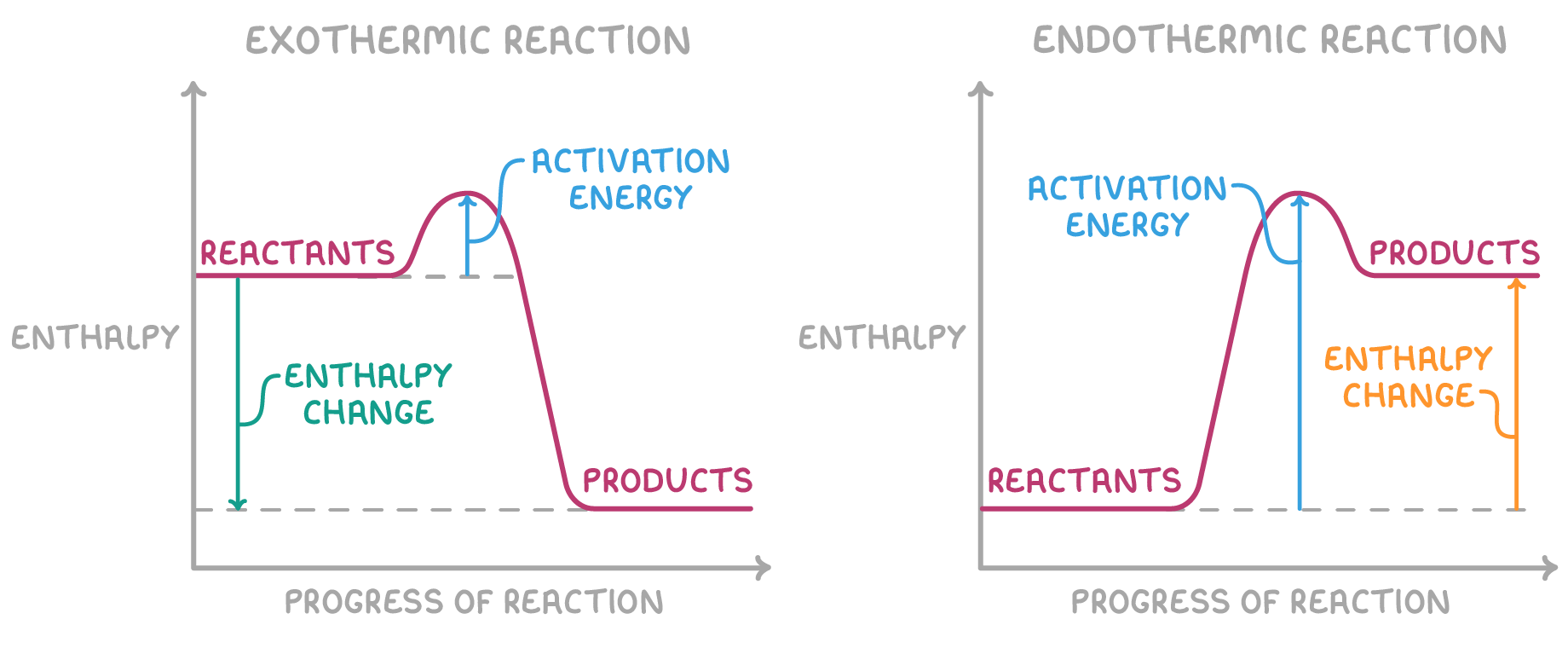
Key features include:
- The activation energy is the minimum energy needed for a reaction to occur.
- In exothermic reactions, the reactants have a higher energy than the products and the ΔH arrow points upwards
- In endothermic reactions, the reactants have a lower energy than the products and the ΔH arrow points downwards
Substances are more stable when they have less enthalpy.
Why standard conditions are used
Actual enthalpy cannot be directly measured, only the change.
Enthalpy changes are measured under standard conditions:
- 298 K temperature
- 101 kPa pressure
The superscript plimsoll (⦵) is used after ΔH to denote that the enthalpy change refers to standard conditions. For example, ΔH⦵r refers to the standard enthalpy change of reaction.
The standard state of a substance is its physical state under standard conditions.
Types of standard enthalpy change
You need to know the definitions for these four types of standard enthalpy change:
- Standard enthalpy change of reaction (ΔH⦵r) - Enthalpy change when a reaction occurs in the molar quantities shown in the chemical equation, under standard conditions
- Standard enthalpy change of formation (ΔH⦵f) - Enthalpy change when 1 mole of a compound forms from elements under standard conditions
- Standard enthalpy change of combustion (ΔH⦵c) - Enthalpy change when 1 mole of a substance combusts fully in oxygen under standard conditions
- Standard enthalpy change of neutralisation (ΔH⦵neut) - Enthalpy change when an acid and alkali neutralise under standard conditions, to form 1 mole of water
Using standard conditions means these values will be the same regardless of who measures them.