Synthetic Routes
This lesson covers:
- What synthetic routes are and why they are important
- The key organic reactions covered so far
- How functional groups determine reactivity
Synthetic routes map out synthetic pathways
Chemists create complex compounds, which are often used in medicines or agricultural chemicals.
Typically, it's not feasible to synthesise the target compound directly from a single starting material in one step.
A synthetic route outlines the sequence of reactions needed to transform starting materials into the desired end product.
It details:
- Every chemical intermediate formed along the way.
- The specific reagents and conditions required at each stage of the process.
For instance, converting 2-chloropropane to propanone isn't a straightforward, one-step process.
Instead, a two-step synthetic route is necessary:
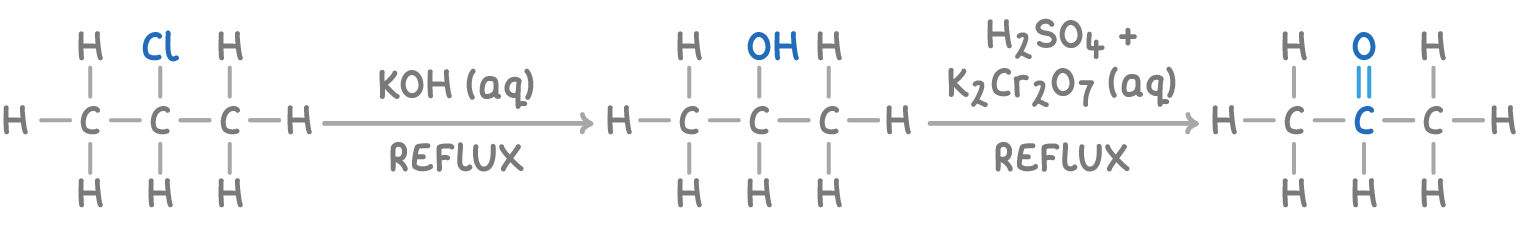
- 2-chloropropane reacts with KOH, producing propan-2-ol.
- Propan-2-ol, when treated with acidified K2Cr2O7, yields propanone.
Summary of key organic reactions
Below is an overview of the primary types of organic reactions we've discussed:
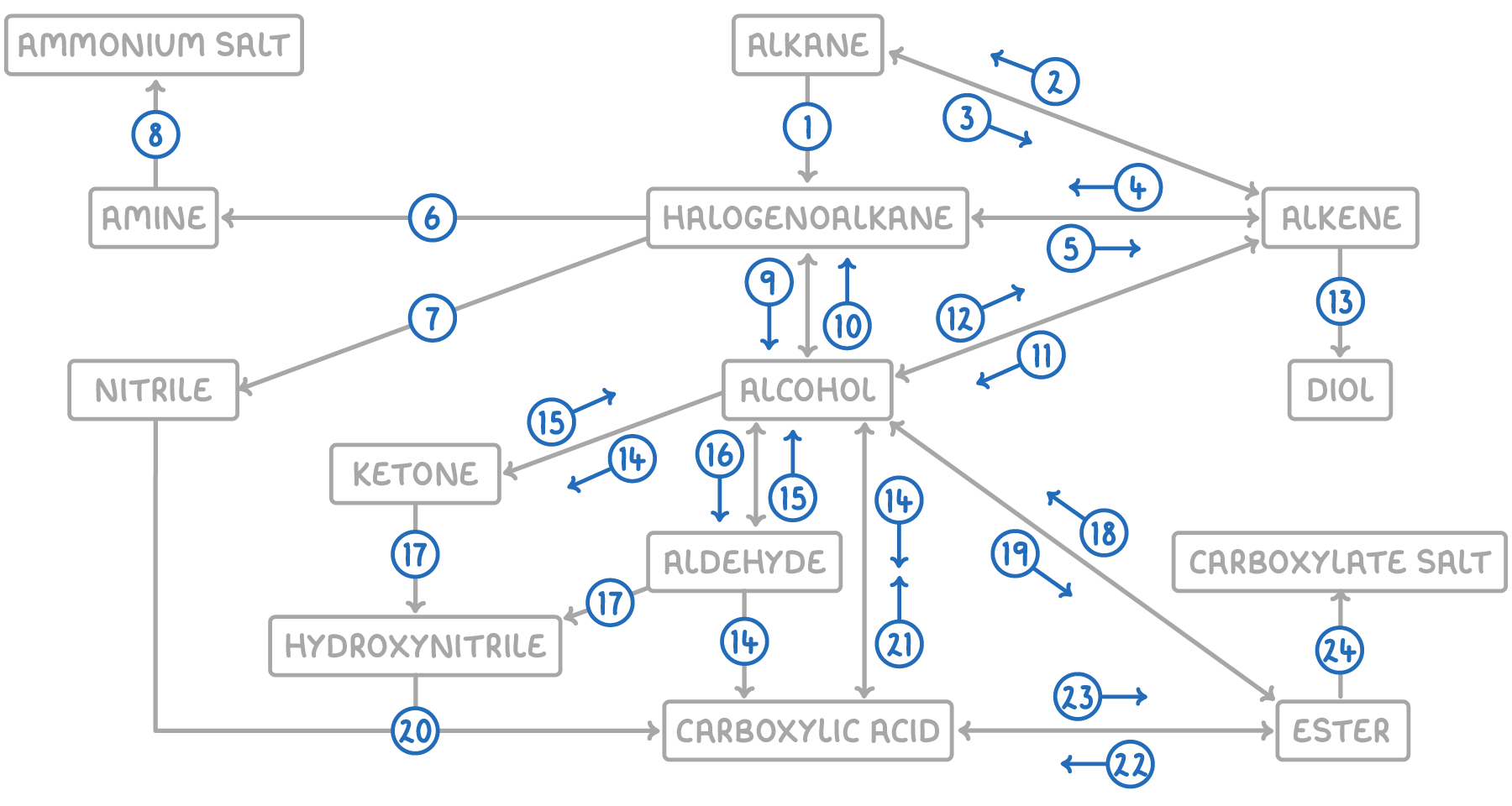
| Reaction | Reagent and conditions | Reaction type |
|---|---|---|
| 1 | Halogen, UV light | Free radical substitution |
| 2 | H2, Ni or Pt catalyst, heat | Electrophilic addition |
| 3 | Al2O3 catalyst, heat | Thermal decomposition |
| 4 | Hydrogen halide or halogen | Electrophilic addition |
| 5 | NaOH in ethanol, reflux | Elimination |
| 6 | Conc. NH3 in ethanol, heat under pressure | Nucleophilic substitution |
| 7 | KCN in ethanol, reflux | Nucleophilic substitution |
| 8 | Dilute HCl(aq) | Acid-base |
| 9 | NaOH(aq), reflux | Nucleophilic substitution |
| 10 | Hydrogen halide, PCl5 or SOCl2 OR KCl and conc. acid OR PCl3 and heat | Nucleophilic substitution |
| 11 | Steam, H3PO4 catalyst, 300°C, 60 atm | Electrophilic addition |
| 12 | Conc. H2SO4 or Al2O3 catalyst, heat | Elimination |
| 13 | Cold, dilute acidified KMnO4(aq) | Oxidation |
| 14 | K2Cr2O7(aq) or KMnO4(aq), H2SO4 catalyst, reflux | Oxidation |
| 15 | NaBH4(aq) or LiAlH4 in dry ether, heat | Nucleophilic addition |
| 16 | K2Cr2O7(aq) or KMnO4(aq), H2SO4 catalyst, distill | Oxidation |
| 17 | HCN, KCN catalyst, heat | Nucleophilic addition |
| 18 | Dilute HCl(aq) or dilute NaOH(aq), heat | Hydrolysis |
| 19 | Carboxylic acid, conc. H2SO4 | Condensation |
| 20 | H2O, dilute HCl(aq), heat | Hydrolysis |
| 21 | LiAlH4 in dry ether, heat | Reduction |
| 22 | Dilute HCl(aq), heat | Hydrolysis |
| 23 | Alcohol, conc. H2SO4 | Condensation |
| 24 | Dilute NaOH(aq) | Acid-base |
Functional groups dictate reactivity
The behaviour and properties of functional groups significantly affect molecular reactions.
For example:
- Nucleophiles are attracted to the partially positive carbon in halogenoalkanes, but they don't target the electron-rich C=C double bond in alkenes.
- Alcohols, due to their polar O-H bond and the lone pair of electrons on the oxygen atom, can act as nucleophiles.
The following table outlines common reactions associated with key functional groups:
| Homologous series | Functional group | Properties | Typical reactions |
|---|---|---|---|
| Alkane | C-C | Nonpolar, unreactive | Free radical substitution |
| Alkene | C=C | Nonpolar, electron-rich | Electrophilic addition, oxidation |
| Alcohol | O-H | Polar O-H bond | Nucleophilic substitution, elimination, oxidation, condensation |
| Halogenoalkane | C-X | Polar C-X bond | Nucleophilic substitution, elimination |
| Aldehyde/Ketone | C=O | Polar C=O bond | Oxidation (aldehydes only), reduction, nucleophilic addition |
| Carboxylic acid | COOH | Electron deficient C | Condensation, reduction |
| Ester | RCOOR’ | Electron deficient C | Hydrolysis |
Compounds with multiple functional groups require the identification of all such groups to accurately predict their reactivity.