Amines
This lesson covers:
- The structure and classification of amines
- How the structure of amines affects their basicity
Amines are derivatives of ammonia
Amines are organic compounds that come from ammonia (NH3). This transformation happens when one or more hydrogen atoms in the ammonia molecule are replaced with alkyl or aryl groups.
Amines can be classified into two main categories: aliphatic and aromatic. Aliphatic amines have alkyl groups attached to the nitrogen atom, while aromatic amines contain a nitrogen atom directly bonded to a benzene ring.
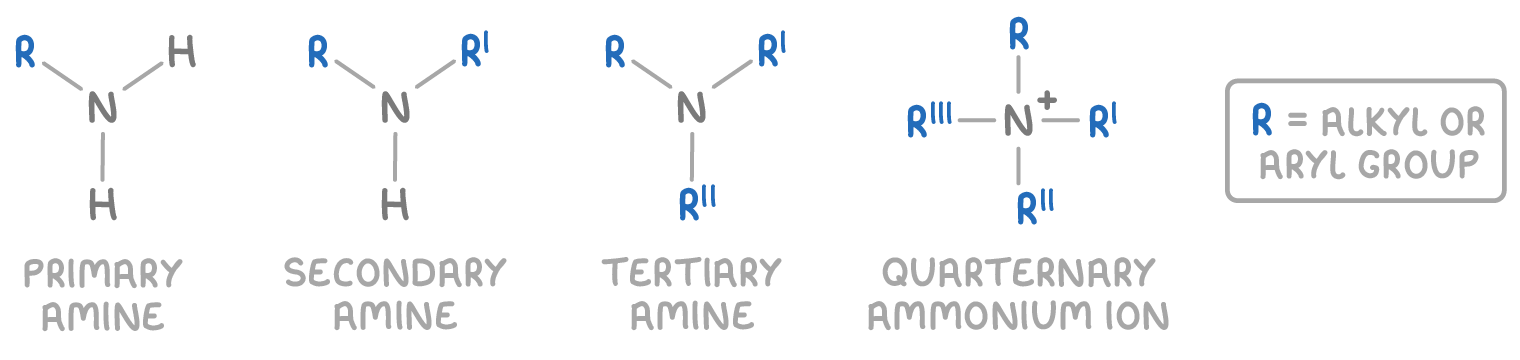
Amines are further categorised based on the number of hydrogen atoms that have been replaced:
- Primary amines - One hydrogen is replaced. The general formula is RNH2.
- Secondary amines - Two hydrogens are replaced. The general formula is R2NH.
- Tertiary amines - Three hydrogens are replaced. The general formula is R3N.
- Quaternary ammonium ions - Four organic groups are attached to positively charged nitrogen, general formula R4N+.
Amines contain a lone pair of electrons
The nitrogen atom in amines possesses a lone pair of electrons, which plays a crucial role in their chemical reactivity and structure. This lone pair results in a trigonal pyramidal geometry with a bond angle slightly less than 109.5°.
Amines act as weak Brønsted-Lowry bases due to their ability to accept protons (H+ ions) using the lone pair. Amines react with acids by accepting H+ ions to form ammonium salts.
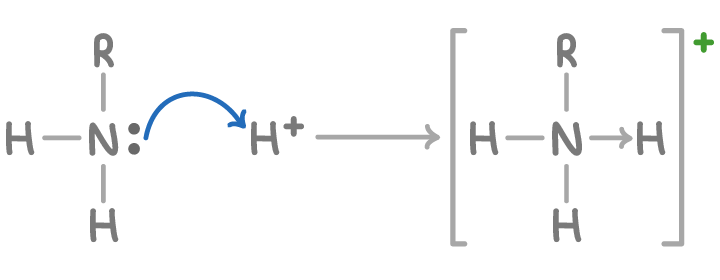
For example, methylamine (CH3NH2) neutralises hydrochloric acid (HCl) to form methylammonium chloride:
CH3NH2 + HCl ➔ CH3NH3+Cl-
The lone pair also makes amines nucleophilic, allowing them to attack electron-deficient species like halogenoalkanes in nucleophilic substitution reactions.
Aliphatic amines are stronger bases than aromatic amines
The strength of an amine as a base is influenced by how available nitrogen's lone pair of electrons is:
- Aromatic amines have the electron density of the nitrogen reduced by the partial delocalisation of the lone pair into the π-system, which decreases its availability for bonding.
- Aliphatic amines, however, have electron-donating alkyl groups that increase the electron density on the nitrogen, making the lone pair more readily available for bonding.
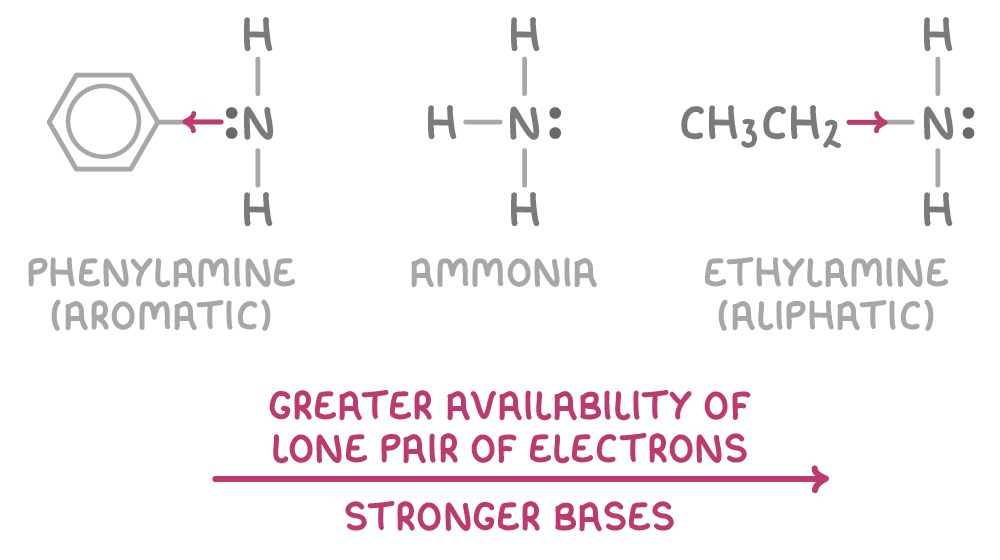
The more available the lone pair of electrons, the stronger the base. So the order of increasing basicity is:
primary aromic amines < ammonia < primary aliphatic amines