The Homologous Series of Alcohols
This lesson covers:
- The functional group and general formula of alcohols
- How alcohols are classified as primary, secondary or tertiary
- How alcohols form hydrogen bonds
- Comparing the acidity of alcohols to water
- Reactions to produce alcohols
Alcohols contain the hydroxyl functional group
Alcohols are organic compounds that contain a hydroxyl (-OH) functional group bonded to a carbon atom.
The general formula for alcohols is:
CnH2n+1OH
Where 'n' represents the number of carbon atoms.
Alcohols are named by replacing the ending "-e" of the corresponding alkane with "-ol", for example:
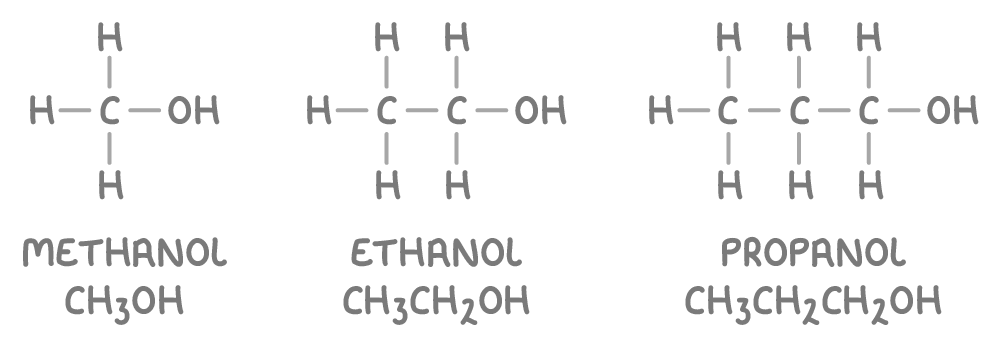
Where neccessary, a numerical prefix to the name is added to indicate the position of the hydroxyl group, for example, propan-1-ol.
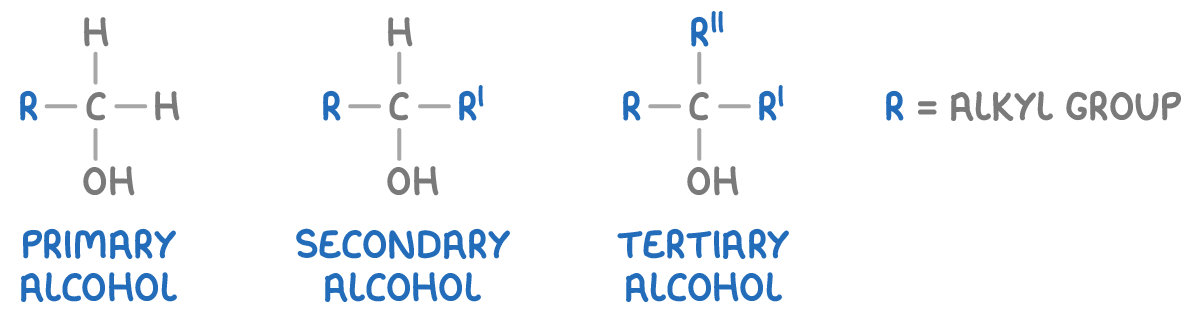
Classification of alcohols
Alcohols are categorised into three types based on the carbon atom to which the hydroxyl group is attached:
- Primary alcohols - The -OH group is attached to a carbon atom that is bonded to only one alkyl group (or no alkyl groups).
- Secondary alcohols - The -OH group is attached to a carbon atom that is bonded to two alkyl groups.
- Tertiary alcohols - The -OH group is attached to a carbon atom that is bonded to three alkyl groups.
Alcohols form hydrogen bonds
The bond between oxygen and hydrogen in the hydroxyl group of alcohols is polar. This is due to oxygen being more electronegative than hydrogen, leading to an unequal sharing of electrons. As a result, the hydrogen atom acquires a partial positive charge (δ+), while the oxygen atom gains a partial negative charge (δ-).
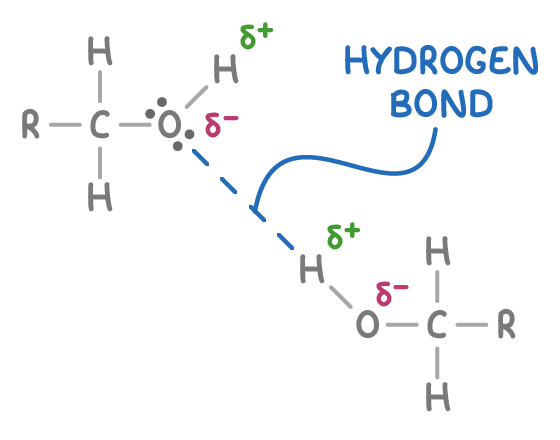
The partially positively charged hydrogen atom (δ+) can form intermolecular hydrogen bonds with the lone pairs of electrons on oxygen atoms found in other polar molecules, including water molecules or other alcohol molecules (as shown above).
Alcohols are weaker acids than water
Alcohols like ethanol are weaker acids than water.
When dissolved in water, ethanol molecules only dissociate to a very small degree:
C2H5OH(aq) ⇌ C2H5O-(aq) + H+(aq)
The equilibrium lies far to the left, with the vast majority of ethanol molecules remaining undissociated.
In contrast, water dissociates to a greater extent:
H2O(l) ⇌ H+(aq) + OH-(aq)
The concentration of H+ ions produced by ethanol dissociation is significantly lower than that produced by water dissociation. This indicates that alcohols are less prone to dissociation and H+ ion formation, making them weaker acids than water.
The lower acidity of alcohols is due to the electron-donating nature of the alkyl group (like ethyl) attached to the negatively charged oxygen in the ethoxide ion. The positive inductive effect of the alkyl group:
- Concentrates more negative charge on the oxygen atom.
- Results in the ethoxide ion has a greater affinity for H+ ions than the hydroxide ions from water dissociation, which only have a hydrogen atom bonded to the negatively charged oxygen.
Reactions to produce alcohols
Alcohols can be produced via several reactions:
- Electrophilic addition of steam to an alkene in the presence of an H3PO4 catalyst. For example, ethene reacts with steam to form ethanol:
CH2=CH2 + H2O(g) ➔ CH3CH2OH
- Reaction of alkenes with cold dilute acidified potassium manganate(VII) to form a diol. For example, ethene reacts with cold dilute acidified KMnO4 to form ethane-1,2-diol:
CH2=CH2 + H2O + [O] ➔ CH2OH-CH2OH
- Substitution of a halogenoalkane using NaOH(aq) and heat. For example, bromoethane reacts with sodium hydroxide to form ethanol:
CH3CH2Br + NaOH ➔ CH3CH2OH + NaBr
- Reduction of an aldehyde or ketone using NaBH4 or LiAlH4. For example, ethanal can be reduced to ethanol using NaBH4:
CH3CHO + NaBH4 ➔ CH3CH2OH
- Reduction of a carboxylic acid using LiAlH4. For example, ethanoic acid can be reduced to ethanol using LiAlH4:
CH3COOH + LiAlH4 ➔ CH3CH2OH
- Hydrolysis of an ester using dilute acid or dilute alkali and heat. For example, ethyl ethanoate can be hydrolysed to ethanol and ethanoic acid using dilute HCl and heat:
CH3COOC2H5 + H2O ➔ CH3COOH + C2H5OH