Nitrogen Gas, Ammonia and Ammonium Compounds
This lesson covers:
- Why nitrogen gas is unreactive
- The basic properties of ammonia
- The structure and formation of ammonium ions
- The displacement of ammonia from ammonium salts
Lack of reactivity of nitrogen gas
Nitrogen gas (N2) makes up 78% of the air, but shows little chemical reactivity under standard conditions.
The unreactivity of nitrogen is due to:
- The triple covalent bond between the two nitrogen atoms being very strong. It has a bond energy of almost 1,000 kJ mol-1.
- The non-polar nature of N2 means it lacks reactivity and does not easily form compounds.
Nitrogen gas only reacts under extreme conditions, such as in lightning storms. The high temperatures and energies provided can break the strong triple bonds and enable nitrogen to react with oxygen gases.
Basic properties of ammonia
Ammonia (NH3) is commercially produced from the unreactive nitrogen found in air by combining it with hydrogen gas under high pressures and temperatures in the presence of an iron catalyst through the Haber process:
N2(g) + 3H2(g) ➔ 2NH3(g)
The ammonia produced is a polar molecule with a pyramidal shape caused by the lone pair on the nitrogen atom. This lone pair enables ammonia to function as a Brønsted-Lowry base by accepting H+ ions from acids and forming NH4+ ammonium ions:
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH−(aq)
Ammonia is a weak base as it does not completely dissociate in water and the position of equilibrium lies mostly to the left.
Structure and formation of ammonium ions
Ammonium ions (NH4+) are formed when ammonia accepts an H+ ion from an acid using its lone electron pair, forming a dative covalent bond:
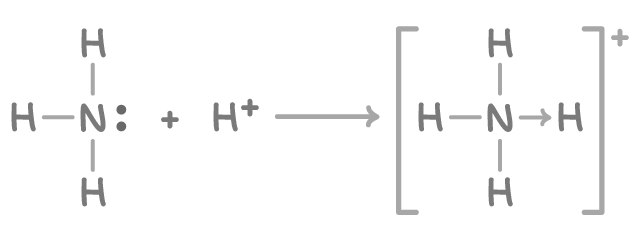
The NH4+ ion has a tetrahedral geometry, with the four bonding pairs of electrons repelling equally to create 109.5° bond angles.
Displacement of ammonia from ammonium salts
Ammonium salts can be decomposed to release NH3 gas by an acid-base displacement reaction.
For example:
2NH4Cl(s) + Ca(OH)2(s) ➔ CaCl2(s) + 2H2O(l) + 2NH3(g)
Here, NH4+ acts as an acid by donating H+ ions, whilst OH- acts as a base by accepting protons. The ammonium ions in the ammonium salt are displaced by calcium ions.
This acid-base reaction is used in the laboratory to generate ammonia gas from ammonium salts, and is the basis of the test for ammonium ions. If an unknown compound contains NH4+ ions, it will produce NH3 gas when heated with a base which turns damp red litmus paper blue.