Electrolysis
This lesson covers:
- What electrolysis is and how it is carried out
- The redox reactions that occur in electrolysis
- How to calculate the amount of substance produced during electrolysis
- How to calculate the Avogadro constant using electrolysis
- Determining the products of electrolysis
Electrolytic cells decompose compounds using electricity
Electrolysis is a process that uses an electric current to break down a compound into its constituent elements. It is commonly employed to extract metals high in the reactivity series, which cannot be obtained by reducing their ores with carbon. Electrolysis also has applications in generating non-metals like chlorine and purifying certain metals.
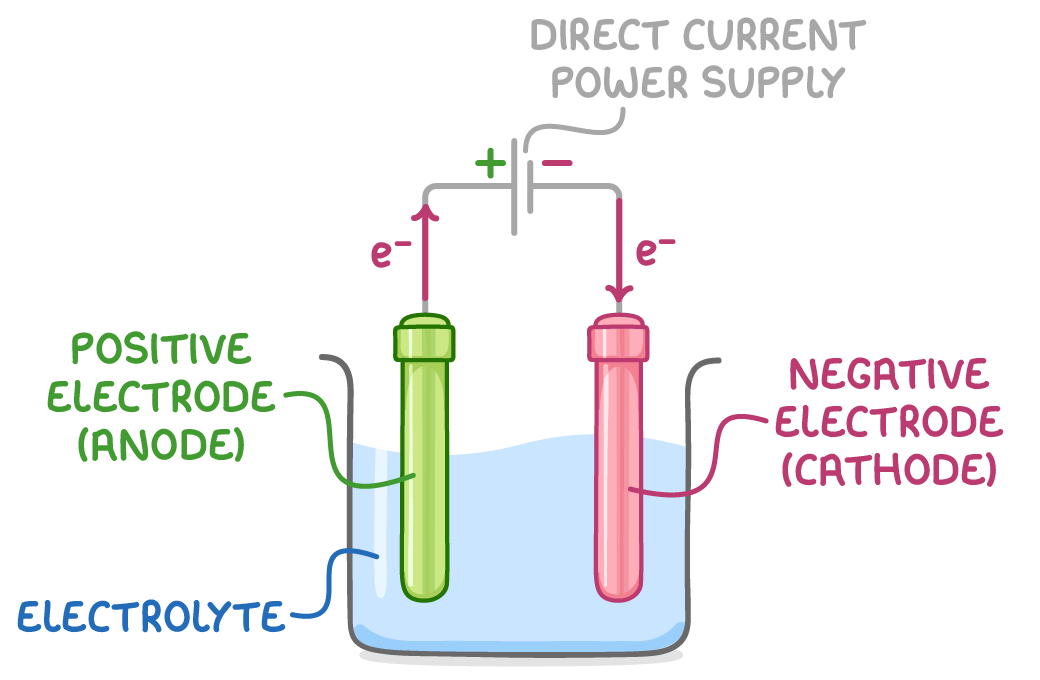
The process of electrolysis takes place in an electrolytic cell, which consists of three main components:
- The electrolyte - This is the compound being decomposed, which can be an aqueous or molten ionic compound.
- Two electrodes - These are conductive rods, usually made of carbon (graphite) or metal, that carry current to and from the electrolyte. The anode is the positive electrode and the cathode is the negative electrode.
- A direct current power supply - This provides the electrical energy necessary to drive the electrolysis process.
Reduction occurs at the cathode, oxidation at the anode
During electrolysis, reduction and oxidation (redox) reactions occur at the electrodes.
At the cathode, positive ions (cations) gain electrons and are reduced, for example:
Cu2+(aq) + 2e- ➔ Cu(s)
At the anode, negative ions (anions) lose electrons and are oxidised, for example:
2Cl-(aq) ➔ Cl2(g) + 2e-
The electron loss at the anode balances the gain at the cathode.
For instance, when molten copper(II) chloride undergoes electrolysis:
Cathode (reduction): Cu2+ + 2e- ➔ Cu
Anode (oxidation): 2Cl- ➔ Cl2 + 2e-
Overall: CuCl2(s) ➔ Cu(s) + Cl2(g)
Calculating the amount of substance deposited during electrolysis
The mass of a substance deposited at an electrode during electrolysis is directly proportional to two factors:
- The duration of the electric current, provided it remains constant.
- The strength of the electric current.
The relationship between the electric current (I), the duration of the current (t), and the total charge passed (Q) can be expressed as:
Q=I×t
Where:
- Q = charge transferred (C)
- I = current (A)
- t = time (s)
To calculate the mass of substance deposited, we need to know the quantity of electricity (charge) passed through the electrolyte. The unit of charge commonly used in electrolysis calculations is the faraday (F). One faraday is defined as the charge carried by one mole of electrons or one mole of singly charged ions, which is equal to 96,500 coulombs per mole (C mol-1).
The faraday is related to the Avogadro constant (L) and the charge of an electron (e) by the equation:
F=L×e
Where:
- F = Faraday's constant (96,500 C mol-1)
- L = Avogadro constant (6.022 × 1023 mol-1)
- e = charge of an electron (1.60 × 10-19 C)
The number of faradays required to deposit one mole of a substance depends on the number of electrons involved in the reduction half-reaction.
For example, in the electrolysis of silver nitrate solution, where silver ions are reduced to solid silver at the cathode:
Ag+(aq) + e- ➔ Ag(s)
One faraday (96,500 C) is needed to deposit one mole of silver, as the reaction involves a single electron.
By contrast, for the electrolysis of copper(II) sulfate solution, where copper ions are reduced to solid copper at the cathode:
Cu2+(aq) + 2e- ➔ Cu(s)
Two faradays (2 × 96,500 C) are required to deposit one mole of copper, as the reduction of one mole of copper ions requires two moles of electrons.
Worked example 1 - Calculating the mass of lead deposited during electrolysis
Calculate the mass of lead deposited at the cathode during electrolysis when a current of 2.00 A flows through molten lead(II) bromide for 30.0 min.
Faraday's constant, F = 96,500 C mol-1.
Step 1: Conversion of minutes to seconds
To covert from minutes to seconds, multiply by 60
30.0 min=1,800 s
Step 2: Equation
Q=I×t
Step 3: Substitution and correct evaluation
Q=2.00×1,800=3,600 C
Step 4: Calculate number of moles of electrons (n)
n=FQ=96,5003,600=0.0373 mol
Step 5: Calculate number of moles of lead deposited
Pb2+(aq) + 2e- ➔ Pb(s)
Pb : e- mole ratio = 1:2
Moles of Pb = 20.0373=0.0187 mol
Step 6: Calculate mass of lead deposited
m = n x Ar = 0.0187 x 207.2 = 3.86 g
Worked example 2 - Calculating the volume of oxygen produced during electrolysis
Calculate the volume of oxygen produced at room temperature and pressure (r.t.p.) when a concentrated aqueous solution of sulfuric acid (H2SO4) is electrolysed for 45.0 min using a current of 0.75 A.
Faraday's constant, F = 96,500 C mol-1.
Step 1: Conversion of minutes into seconds
To convert from minutes into seconds, multiply by 60
45.0 min =2,700 s
Step 2: Equation
Q=I×t
Step 3: Substitution and correct evaluation
Q=0.75×2,700=2,025 C
Step 4: Calculate number of moles of electrons (n)
n=FQ=96,5002,025=0.0210 mol
Step 5: Calculate number of moles of oxygen produced
4OH- ➔ O2 + 2H2O + 4e-
O2 : e- mole ratio = 1:4
Moles of O2 = 40.0210=5.25×10−3 mol
Step 6: Calculate volume of oxygen produced
V = n x Vm = (5.25 x 10-3) x 24 = 0.126 dm3
Determining the Avogadro constant through electrolysis
The Avogadro constant (L), which represents the number of particles in one mole of a substance, can be determined using an electrolytic method.
This involves calculating the charge associated with one mole of electrons using the relationship:
L=charge of one electroncharge of one mole of electrons
The charge on a single electron can be determined experimentally and is approximately 1.60×10−19 C.
To find the charge on one mole of electrons, a simple electrolysis experiment can be conducted using the apparatus shown in the image below.
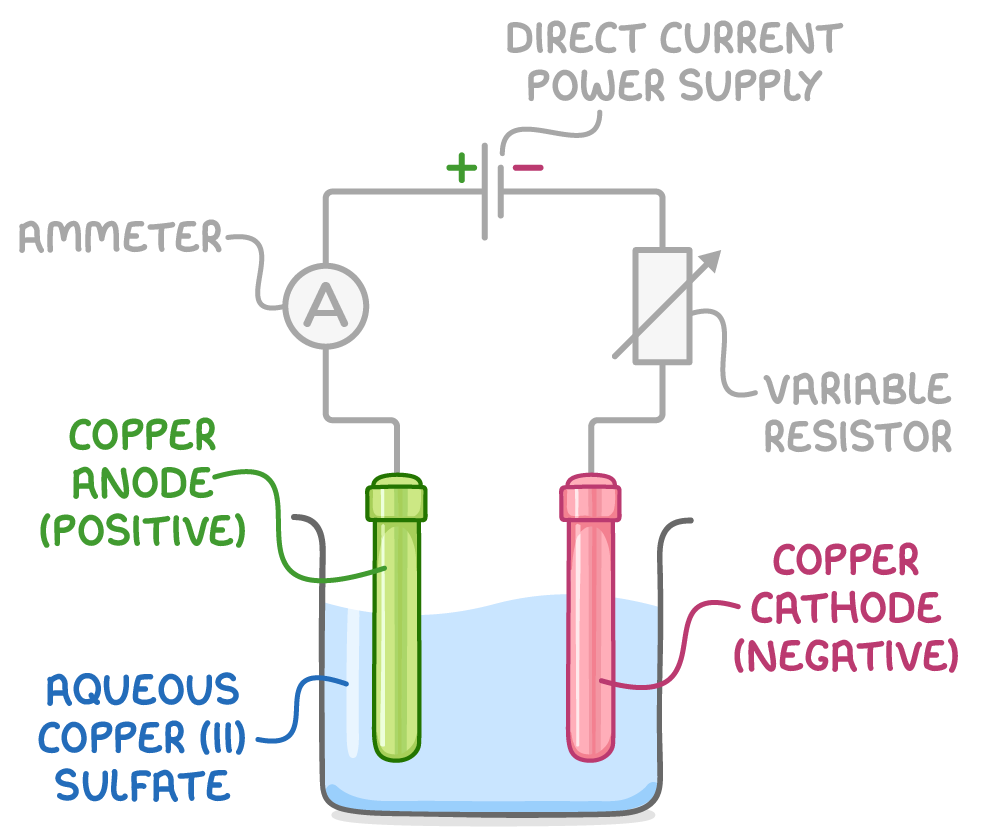
The experimental procedure consists of the following steps:
- Accurately weigh a pure copper anode and cathode separately.
- Set up the apparatus as shown in the image, using a variable resistor to maintain a constant current.
- Pass the constant electric current through the system for a precisely measured time interval.
- Remove the cathode and anode from the setup, wash them with distilled water followed by propanone, and then dry them thoroughly.
- Reweigh the cathode and anode to determine the change in mass.
- Calculate the charge of one mole of electrons using the charge transferred, the change in mass of the anode, and the mole ratio of electrons to copper atoms transferred.
During the experiment, the cathode increases in mass due to the deposition of copper, while the anode decreases in mass as copper dissolves into the solution to form copper ions. It is preferable to measure the decrease in mass of the anode, as the deposited copper may not always adhere well to the cathode surface.
Worked example 3 - Calculating Avogadro's constant using electrolysis
Determine Avogadro's constant using electrolysis data, where a 0.25 A current is applied for 60 min, and the decrease in mass of the copper anode is 0.29 g.
Charge of one electron = 1.60 x 10-19 C
Step 1: Conversion of minutes into seconds
To convert from minutes into seconds, multiply by 60
60 min =3,600 s
Step 2: Equation
Q=I×t
Step 3: Substitution and correct evaluation
Q=0.25×3,600=900 C
Step 4: Calculate number of moles of copper dissolved
n =Arm=63.50.29=4.57×10−3 mol
Step 5: Calculate number of moles of electrons transferred
Cu2+ + 2e- ➔ Cu
e- : Cu mole ratio = 2:1
Moles of e- = (4.57 x 10-3) x 2 = 9.13 x 10-3 mol
Step 6: Calculate charge per mole of electrons
9.13 x 10-3 mol of electrons transferred 900 C of charge
Charge transferred per mole of electrons = nQ=(9.13×10−3)900=98,534 C
Step 7: Calculate Avogadro's constant (L)
L=Charge of one electronCharge of one mole of electrons=(1.60×10−19)98,534=6.16×1023 mol−1
Predicting the products of electrolysis
The products of electrolysis depend on the nature of the electrolyte and the concentration of ions in aqueous solutions.
Electrolysis of molten electrolytes
When pure molten ionic compounds containing two simple ions are electrolysed, a metal is formed at the cathode, and a non-metal is produced at the anode.
The table below shows some examples:
| Molten electrolyte | Cathode product | Anode product |
|---|---|---|
| Aluminium oxide | Aluminium | Oxygen |
| Sodium chloride | Sodium | Chlorine |
| Lead bromide | Lead | Bromine |
Electrolysis of aqueous solutions
Aqueous electrolyte solutions contain multiple cations and anions.
For example, an aqueous sodium chloride solution contains Na+, Cl-, H+, and OH- ions.
The H+ and OH- ions are present due to the ionisation of water:
H2O(l) ⇌ H+(aq) + OH-(aq)
The ions discharged during the electrolysis of aqueous solutions depend on two main factors:
- The relative electrode potentials of the ions
- The concentration of the ions in the solution
Cathode reactions
At the cathode, the cation that is most easily reduced (i.e., has the most positive reduction potential) is discharged.
For instance, in a concentrated aqueous NaCl solution (1.00 mol dm-3), H+ ions are discharged at the cathode instead of Na+ ions because they have a more positive standard electrode potential (E⦵):
H+(aq) + e- ➔ ½H2(g) E⦵ = 0.00 V
Na+(aq) + e- ➔ Na(s) E⦵ = -2.71 V
Anode reactions
At the anode, when using platinum electrodes, the ease of oxidation of anions follows the electrochemical series:
SO42-(aq) < NO3-(aq) < Cl-(aq) < OH-(aq) < Br-(aq) < I-(aq)
For example, in a concentrated aqueous Na2SO4 solution, OH- ions are discharged at the anode instead of SO42- ions because they are more easily oxidised:
4OH-(aq) ➔ O2(g) + 2H2O(l) + 4e-
Effect of ion concentration
The concentration of ions in the solution also plays a role in determining which species are discharged.
For example, in a concentrated NaCl solution, Cl- ions are oxidised at the anode instead of OH- ions, even though they are lower in the electrochemical series. This is due to the much higher concentration of Cl- ions compared to OH- ions:
2Cl-(aq) ➔ Cl2(g) + 2e-
However, when the NaCl solution is extremely dilute, the lower concentration of Cl- ions leads to the formation of O2 at the anode from the oxidation of OH- ions:
4OH-(aq) ➔ O2(g) + 2H2O(l) + 4e-