Reactions of the Alcohols
This lesson covers:
- Combustion of alcohols
- Oxidation of alcohols
- Structures of aldehydes, ketones and carboxylic acids
- Substitution of alcohols to produce halogenoalkanes
- Dehydration of alcohols to produce alkenes
- Esterification of alcohols
- Reaction with sodium
Complete combustion of alcohols
Alcohols combust completely when burned in an excess of oxygen, breaking all C-C and C-H bonds. This results in the production of carbon dioxide and water, along with the release of heat energy.
For example, the complete combustion of ethanol is represented by the equation:
C2H5OH(l) + 3O2(g) ➔ 2CO2(g) + 3H2O(g)
Oxidation of alcohols using acidified K2Cr2O7 or KMnO4 solutions
Oxidation of alcohols can be performed using a potassium dichromate(VI) solution (K2Cr2O7), acidified with dilute sulfuric acid. The solution changes colour from orange to green as the reaction proceeds due to the reduction of dichromate(VI) ions (Cr2O72-) to chromium(III) ions (Cr3+).
Alcohols can also be oxidised using acidified potassium permanganate(VII) (KMnO4), which changes colour from purple to colourless as the reaction proceeds. This colour change is due to the reduction of permanganate(VII) ions (MnO4-) to manganese(II) ions (Mn2+).
The extent of oxidation depends on the alcohol's structure:
- Primary alcohols are oxidised to aldehydes and then to carboxylic acids.
- Secondary alcohols are oxidised to ketones only.
- Tertiary alcohols do not oxidise under these conditions.
Controlling oxidation of primary alcohols
The oxidation of primary alcohols can proceed in two stages, initially forming an aldehyde and subsequently a carboxylic acid:
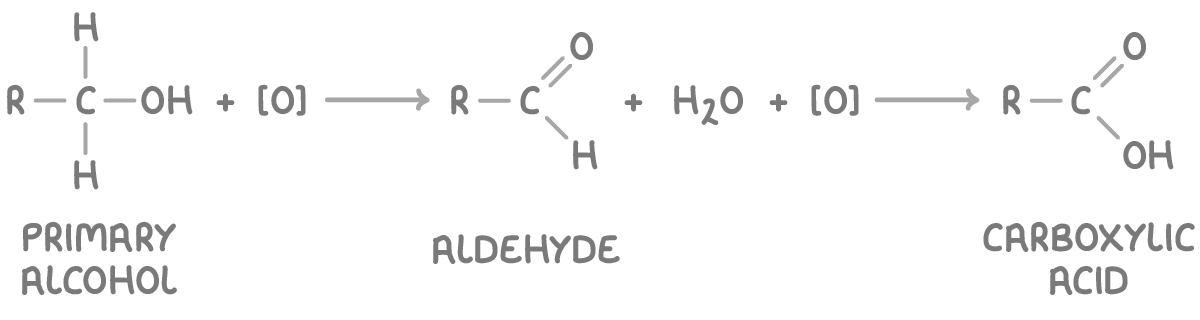
Here, [O] represents an oxidising agent.
To isolate the aldehyde, gently heat the alcohol in a distillation apparatus with a limited amount of potassium dichromate(VI) and distil the aldehyde as it forms to prevent further oxidation. To obtain the carboxylic acid, heat the alcohol with an excess of dichromate(VI) under reflux conditions.
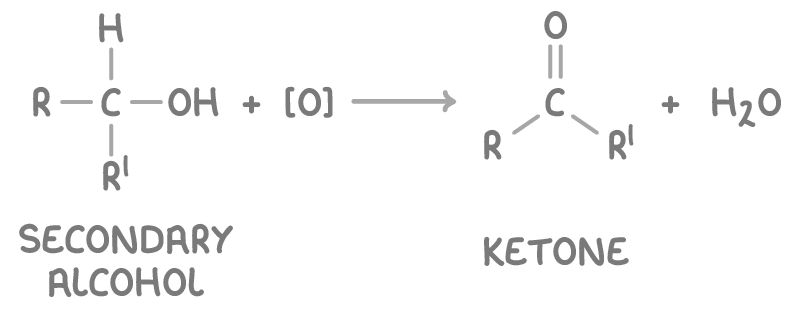
Oxidising secondary alcohols
Secondary alcohols, such as propan-2-ol, can be converted into ketones by refluxing with acidified dichromate(VI).
This process does not allow for further oxidation of the ketone:
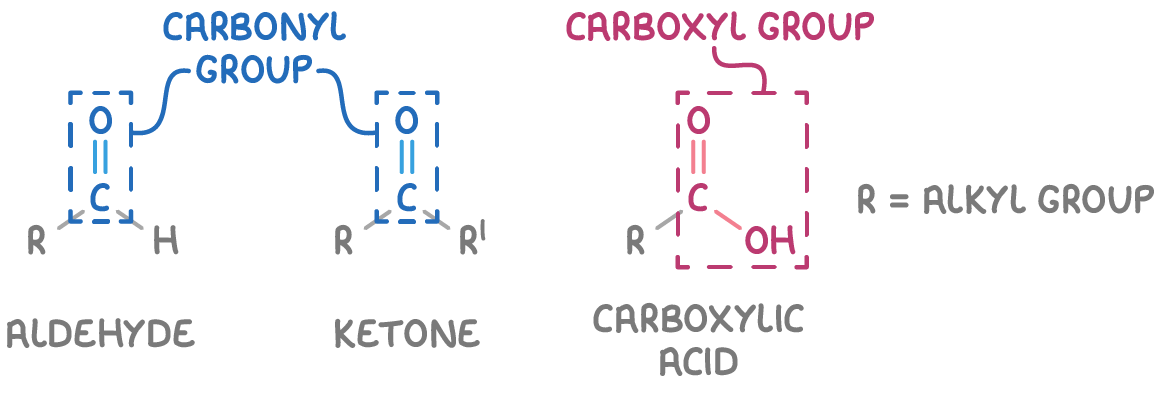
Structure of aldehydes, ketones and carboxylic acids
Aldehydes and ketones are characterised by the presence of a carbonyl functional group (C=O), but differ in their structure:
- Aldehydes have a hydrogen atom and an alkyl group attached to the carbonyl carbon.
- Ketones have two alkyl groups attached to the carbonyl carbon.
Carboxylic acids feature a carboxyl functional group (COOH) attached to an alkyl group.
Converting alcohols to halogenoalkanes
Alcohols can undergo substitution reactions to form halogenoalkanes using various reagents:
- Reaction with hydrogen halides (HX) or sodium halides and concentrated acids:
- Alcohols can react directly with hydrogen halides, such as HCl or HBr, or with sodium halides (e.g., NaCl or KBr) in the presence of concentrated sulfuric acid (H2SO4) or phosphoric acid (H3PO4).
- When using sodium halides and concentrated acids, the acid generates the hydrogen halide in situ, which then reacts with the alcohol.
- These reactions replace the -OH group with a halogen atom, producing a halogenoalkane and water. Examples:
C2H5OH + HCl ➔ C2H5Cl + H2O
C2H5OH + NaCl + H2SO4 ➔ C2H5Cl + NaHSO4 + H2O
- Reaction with phosphorus chlorides:
- Alcohols can react with phosphorus halides, such as PCl3 or PCl5, to form halogenoalkanes.
- These reactions may require heating and produce acidic byproducts. Examples:
C2H5OH + PCl5 ➔ C2H5Cl + HCl + POCl3
3C2H5OH + PCl3 ➔ 3C2H5Cl + H3PO3 (requires heating)
- Reaction with thionyl chloride (SOCl2):
- Alcohols can be converted to chloroalkanes using thionyl chloride.
- This reaction produces gaseous byproducts (HCl and SO2), which escape from the reaction mixture. Example:
C2H5OH + SOCl2 ➔ C2H5Cl + HCl + SO2
Dehydrating alcohols to form alkenes
Dehydration of alcohols, an elimination reaction facilitated by a heated catalyst such as Al2O3 or concentrated H2SO4, results in the formation of alkenes through the elimination of water:
alcohol ➔ alkene + water
For instance, dehydrating ethanol with concentrated sulfuric acid produces ethene:
CH3CH2OH ➔ CH2=CH2 + H2O
Esterification of alcohols
Esterification is a condensation reaction that involves reacting alcohols with carboxylic acids in the presence of a concentrated H2SO4 catalyst to form esters:
alcohol + carboxylic acid ⇌ ester + water
An example is the reaction of ethanol with ethanoic acid to produce ethyl ethanoate:
C2H5OH + CH3COOH ⇌ CH3COOC2H5 + H2O
Reaction of alcohols with sodium
Alcohols react with sodium metal to form sodium alkoxides and hydrogen gas:
alcohol + sodium ➔ sodium alkoxide + hydrogen
For example, ethanol reacts with sodium to form sodium ethoxide and hydrogen gas:
C2H5OH + Na ➔ C2H5ONa + 1⁄2H2